-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2015; 4(2): 21-27
doi:10.5923/j.medicine.20150402.01
Effect of Tamoxifen and Flutamide-Induced Receptor Blockade on Bisphenol a (BPA) Activity in Male Albino Wistar Rats
Tunmise T. Makinwa , Patrick O. Uadia
Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Nigeria
Correspondence to: Tunmise T. Makinwa , Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Bisphenol A (BPA) is one of the endocrine disrupting chemicals. Its estrogenic properties are established; however studies on the effects of BPA on androgen receptors are sparse. We compared the effects of blocking the androgen and estrogen receptors on BPA mechanisms of action in albino rats. Fifty four male albino rats were grouped into nine; group 1 received the vehicle (corn oil), group 2 received flutamide and BPA (100µg/kg/day), group 3 received flutamide and BPA (4mg/kg/day), group 4 received tamoxifen and BPA (100µg/kg/day), group 5 received tamoxifen and BPA (4mg/kg/day), group 6 received BPA (100µg/kg/day) only, group 7 received BPA (4m/kg/day) only, group 8 received flutamide only and group 9 received tamoxifene only. Exposure of rats to a single low (100µg/kg) and high (4mg/kg) dose of BPA induced a rapid decrease in glycemia; which was inhibited by pre-treatment with flutamide but pre-treatment with tamoxifen showed no inhibitory effect. Conversely long-term (12days) injection of BPA (low (100µg/kg/day) and high (4mg/kg) doses) resulted in hyperglycemia. The BPA-induced hyperglycemia was significantly (P<0.05) inhibited by pre-treatment with tamoxifen. Although flutamide injection inhibited the BPA-induced hyperglycemia the effect was not significant (P>0.05). Long-term exposure to high dose (4mg/kg) of BPA resulted in a significant (P<0.05) decrease in serum testosterone compared with the control groups, this effect was not blocked by either flutamide or tamoxifen. This study revealed that both estrogen and androgen receptors are mediators of BPA’s action on glucose metabolism and reproduction.
Keywords: Endocrine disruptor, Bisphenol A, Tamoxifen, Flutamide, Androgen receptor and Estrogen receptor
Cite this paper: Tunmise T. Makinwa , Patrick O. Uadia , Effect of Tamoxifen and Flutamide-Induced Receptor Blockade on Bisphenol a (BPA) Activity in Male Albino Wistar Rats, Basic Sciences of Medicine , Vol. 4 No. 2, 2015, pp. 21-27. doi: 10.5923/j.medicine.20150402.01.
Article Outline
1. Introduction
- The endocrine system of the body regulates metabolic processes including nutrition, behavioural, reproductive, growth, cardiovascular and kidney function [1]. All the endocrine systems of the body are integrated. The result is that environmental compounds may affect several endocrine systems such as, developmental, immune, reproductive, cardiovascular and neurological function [2]. Environmental compounds that can affect the endocrine system are called endocrine disruptors (EDs). Most EDs interfere with the reproductive system by interfering with signalling, some either inhibit synthesis or interfere with the metabolism of the sex steroid hormones [3]. Most acting as either agonists or antagonist of the steroidal sex hormones estrogens and androgens by binding to their receptors and in so doing may stimulate or inhibit the transcriptional or post-translational mechanisms [3]. Moreover EDs effects may be mediated through membrane bound receptors or ion channels. Whatever the mechanisms EDs may interfere with the production, release, transport, metabolism, binding, action, or elimination of natural hormones in the body responsible for the maintenance of homeostasis and the regulation of developmental processes [4].Bisphenol A (BPA) is one of the 230 chemicals considered as endocrine disruptors [5]. Studies have shown that BPA is estrogenic [6, 7]. BPA exerts its actions by binding to both estrogen receptors ERα and ERβ [8]. It was revealed that BPA also targets the insulin producing beta cell in the pancreas and provokes beta cell exhaustion as well as insulin resistance [9], through the estrogen receptors (ER) [10], by mimicking the actions of estradiol on blood glucose homeostasis and over stimulating the estrogen receptors. Other studies have shown that BPA at low nanomolar concentrations inhibited the release of adiponectin from isolated human adipocytes and stimulated the release of interleukin-6 (IL-6) and tumor necrosis factor (TNFα), a direct effect of BPA that would be predicted to diminish insulin sensitivity [11]. Although there are studies reporting the anti androgenic effects of BPA [12, 13], most of these studies are in vitro studies. It was therefore, worthwhile to investigate and compare the effects of BPA, on glucose metabolism and testosterone level in male rats, in the presence and absence of androgen or estrogen receptor blockers.
2. Materials and Methods
2.1. Drugs and Chemicals
- All reagents were of analytical grade. Insulin was obtained from LILLY PHAM, EGYPT, flutamide from Osake pharmaceutical PVT LTD, India, Tamoxifene from PL Holder: Generics UK Limited, Bisphenol A from Sigma-Aldrich. USA and, Corn oil from Mazola corn oil, USA.
2.2. Animals
- Fifty four male albino Wistar rats (8 weeks old) were obtained from the Department of Biochemistry animal house, University of Benin, Benin City, Nigeria. All experimental animals were treated humanely according to the guideline of animal care/ animal house of our university. They were maintained under standard conditions and were fed pelletized animal grower marsh obtained from Vital Animal feeds limited, Nigeria, ad libitum. Animals received their drinking water in metal containers to reduce exposure to BPA in plastic feeding bottles.
2.3. Experimental Protocol
- The animals were randomly divided into nine groups of six animals each as follow;Group 1: control rats, received sc injection of 0.5ml/kg/day Corn oil for 24 days. Group 2: was pre-treated with im injection of Flutamide (FM) (12 mg/kg/day) as described by Montalvo et al. for 12 days [14] and on the next day was given sc injection of BPA (100µg/kg/day) as described by Alonso-Magdalena et al. for 12 days [15]. Group 3: was pre-treated with im injection of Flutamide (FM) (12 mg/kg/day) for 12 days and on the next day was given sc injection of BPA (4mg/kg/day) for 12 days.Group 4: was pre-treated with im injection of Tamoxifen (TM) (0.6 mg/kg/day) as described by James et al. for 12 days [16] and on the next day was given sc injection of BPA (100µg/kg/day) for 12 days.Group 5: was pre-treated with im injection of Tamoxifen (TM) (0.6 mg/kg/day) for 12 days and on the next day was given sc injection of BPA (4mg/kg/day) for 12 days.Group 6: received sc. injection of (0.5ml/kg/day) Corn oil for 12 days and on the next day was given sc injection of BPA (100µg/kg/day) for 12 days. Group 7: received sc injection of (0.5ml/kg/day) Corn oil for 12 days and on the next day was given sc injection of BPA (4mg/kg/day) for 12 days.Group 8: was pre-treated with im injection of flutamide (FM)(12 mg/kg/day) for 12 days and on the next day was given sc injection of Corn oil (0.5ml/kg/day) for 12 days.Group 9: was pre-treated with im injection of Tamoxifen (TM) (0.6 mg/kg/day) for 12 days and on the next day was given sc injection of Corn oil (0.5ml/kg/day) for 12 days.Flutamide and Tamoxifen were dissolved in 5% ethanol mixed with corn oil 1:9 vol/vol. BPA was dissolved in 5% ethanol mixed with corn oil 1: 9 vol/vol. The amount of vehicle was kept constant for all the groups.
2.4. Glycemia Determination
- On the 13th day after 12 days of pre-treatment with receptor blockers (Flutamide and Tamoxifen), blood samples were drawn from fed animals by tail cut before the subcutaneous injection of BPA at 0 min and after BPA injection at 15, 30, 45, 60 and 120 min for the determination of blood glucose, using an Accu-Chek compact glucometer (Roche, Madrid, Spain).
2.5. Insulin Tolerance Tests
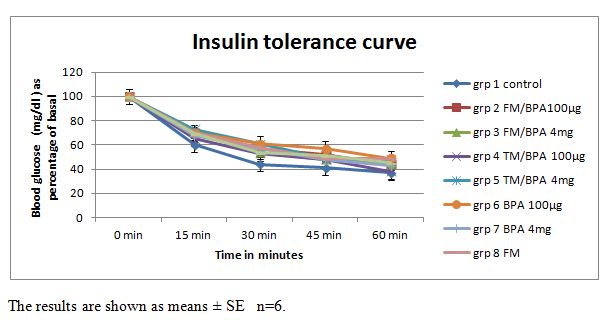
- Insulin tolerance in experimental animals was determined after 11 days of subcutaneous injection of BPA at low and high dosage with and without flutamide or tamoxifen. For the intraperitoneal insulin tolerance tests (IPITT), fed animals were injected intraperitoneally with soluble insulin at 1.0 IU/kg body weight. Blood glucose was measured in each animal after 0, 15, 30, 45, and 60 min using an Accu-Chek compact glucometer (Roche, Madrid, Spain). The blood glucose value at each point was calculated as a percentage of basal. Then the area under the curve (AUC) (milligrams per deciliter-minute) was calculated using Tai's mathematical model 1994 [17] as an index of insulin tolerance.The animals were killed after 12 days of BPA injection. Blood was collected in plain bottles and bottles containing fluoride oxalate, for serum and plasma. The samples were centrifuged at 4C, the serum and plasma were stored at −20C until determination of hormone concentrations and plasma glucose concentration.
2.6. Biochemical Analysis
- Plasma glucose was determined by glucose oxidase enzymatic colorimetric technique, using kits supplied by RANDOX laboratories limited, UK. Serum testosterone was determined quantitatively by ELISA technique using testosterone (competitive) ELISA kit supplied by Monobind Inc. Lake Forest, CA, USA.
2.7. Statistical Analysis
- Values are expressed as the mean ± SE. Results were statistically analyzed by one-way analysis of variance (ANOVA) for differences between means of different groups. All data were analyzed using SPSS statistical package (SPSS Inc.) version 13.0. A probability of P<0.05 was considered statistically significant.
3. Results
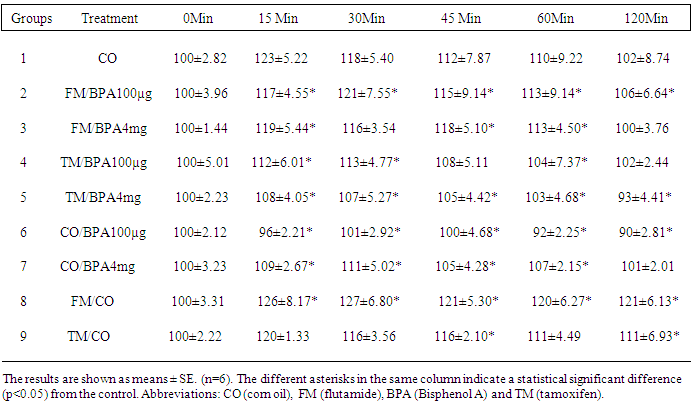
- BPA binds to estrogen and possibly androgen receptors, but it also binds to β-cells of the pancreas stimulating it to release insulinAs shown in table 1: The administration of a single dose of 100μg/kg BPA and 4mg/kg BPA to rats resulted in a significant (P<0.05) decrease in blood glucose concentrations in a time dependent manner, with 100μg/kg being more effective than 4mg/kg compared with the increase in blood glucose of rats in the control group administered only corn oil. Administration of flutamide alone resulted in an increase in the blood glucose than tamoxifen probably because these are male rats with more androgen than estrogen receptors. To determine and evaluate the role of androgen receptor (AR) on BPA mechanisms of action, we compared the effects of AR and ER blockers on the actions of BPA, by treating Albino rats with flutamide and tamoxifen intramuscularly for 12 days before injecting them with BPA. Flutamide treatment prevented the BPA-dependent decrease in blood glucose both at low (100µg/kg) and high (4mg/kg) BPA injection. However tamoxifene had no significant (P>0.05) inhibitory effect both at low dose (100µ/kg) and high dose (4mg/kg) of BPA compared with the BPA treated groups. At each time interval flutamide was more effective in inhibiting the BPA-dependent decrease in blood glucose compared with tamoxifene. This is probably due to more competition for the androgen receptors than the estrogen receptors.
|
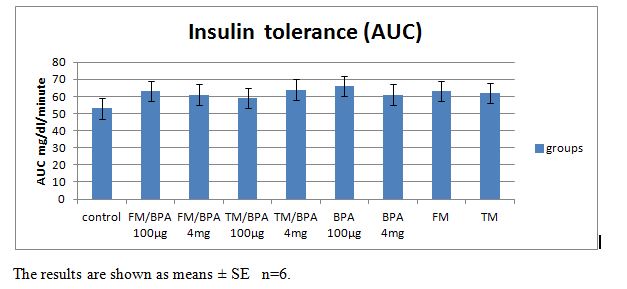 | Figure 2. Showing the area under the curve (AUC) mg/dl/min values in rats injected with the vehicle, 100μg/kg or 4mg/kg body weight BPA, with or without pre-treatment with flutamide or tamoxifen |
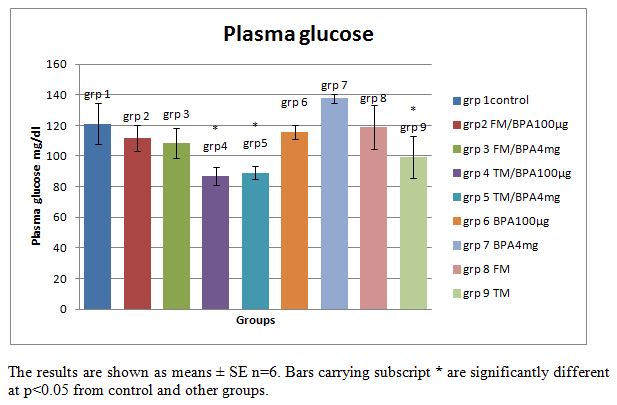 | Figure 3. Plasma glucose (mg/dl) concentration in rats, injected with the vehicle, 100μg/kg or 4mg/kg body weight BPA for 12 days, with or without pre- treatment with flutamide or tamoxifen |
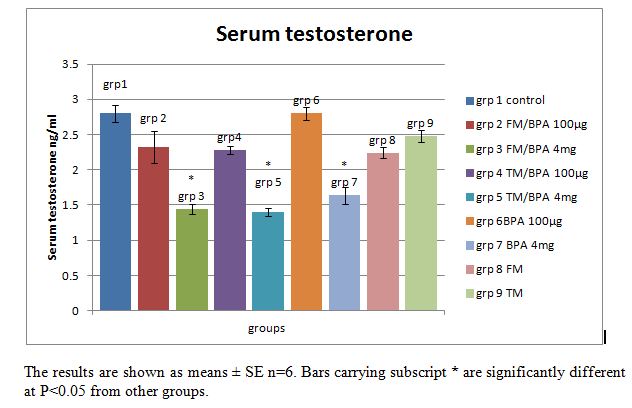 | Figure 4. Serum Testosterone in ng/ml concentration in animals, injected with the vehicle, 100μg/kg or 4mg/kg body weight BPA for 12 days with or without pre-treatment with flutamide or tamoxifen |
4. Discussion
- Estrogen and androgen receptors are much more than sex hormones. It is well established that estrogen and androgen play important roles in fat and carbohydrate metabolism [15, 18]. The biological activities of these sex hormones are mediated by their receptors estrogen receptor (ER) and androgen receptor (AR), key elements in the signal transduction cascade. As shown in Table 1: the injection of a single subcutaneous dose of (high 4mg/kg dose and low 100µg/kg dose) BPA caused a decrease in glycemia in the BPA injected rats, measured at 15 minutes interval for 2 hr compared with the increase in glycemia observed in the control groups who received the vehicle, tamoxifen and flutamide only. This result revealed that BPA may mimic the effect of estradiol (E2) on glucose metabolism. In a previous study by Alonso-madgalena et al., they observed that BPA caused a rapid change in glycaemia by inducing a hypersecretion of insulin as happens with Estradiol, 30 min after the administration of 10 μg/kg of BPA there was an increase in plasma insulin levels, this effect disrupts the pancreatic beta-cell function in vivo and induced insulin resistance [15]. In this study, the BPA-induced hypoglycemic effect was significantly (P<0.05) inhibited by pre-treatment with the androgen receptor antagonist flutamide, conversely the hypoglycemic effect of BPA is unaffected by pre-treatment with Tamoxifene (an estrogen receptor blocker) especially at 100µg/kg. This result shows that while the hypoglycemic effect of BPA may not be AR or ER mediated but rather via another rapid pathway, the level of blood glucose is also dependent on the level of insulin sensitivity of the tissues which determines its uptake into target tissues. As shown in the rapid glycemic test result, the inhibition of the BPA-induced hypoglycemia by flutamide (an androgen receptor blocker) may be due to the blockade of the androgen receptors which may have resulted in the development of insulin resistance in the animals prior to the first BPA injection, since both the ERs and ARs are thought to be involved in maintaining normal insulin sensitivity.Tamoxifen is a selective estrogen receptor modulator [19], i.e. it can act as either an agonist or an antagonist depending on the tissue or the location of the ER. As shown in Fig 3; long term (12days) exposure to BPA induced hyperglycemia in the rats although the level is not significantly (P>0.05) different from the control groups. Pre-treatment with tamoxifen injection significantly (P<0.05) blocked the BPA and fatty acid-induced hyperglycemia in the BPA injected and vehicle treated groups respectively. From the result it is suggested that tamoxifene may have blocked the action of BPA on the pancreatic β-cells or other tissues involved in carbohydrate metabolism such as adipose tissue, hence blocking the BPA-induced hyperglycemia caused by long term exposure. This result revealed that blockage of the long term effects of BPA by tamoxifen is an estrogen receptor (ER) mediated effect. This is similar to a previous study by Alonso-Magdalena et al., they studied the involvement of classical ERs in the regulation of E2 and BPA-induced insulin expression, using mice treated with the anti-estrogen ICI (Fulvestrant). The pure anti-estrogen ICI (Fulvestrant) completely blocked the hyperinsulinemia and subsequently insulin resistance induced after 4 days of both E2 and BPA injection indicating that the action performed on insulin content is mediated by a classical ER [15]. Conversely this present study revealed that pre-treatment with flutamide did not significantly (p<0.05) block the BPA and fatty acid-induced hyperglycemia in the BPA injected and vehicle treated groups. The altered glucose metabolism in these rats was consistent with the fact that these rats had developed insulin resistance as observed in the glycemia results. This may be due to the anti-androgenic effects of flutamide on the androgen receptors. It has previously been reported that while anti-androgens, such has hydroxyl flutamide, have been used to treat AR-dependent cancers for many years, these same treatments can increase the factors that contribute to Type II Diabetes and Coronary heart disease [20]. Moreover several studies have revealed that there is an inverse correlation between serum testosterone level and insulin resistance [18, 21]. Insulin sensitivity is a non-linear process and fluctuations occur during a normal life cycle. Thus diminished insulin sensitivity is observed during pregnancy, puberty or aging, which means that the efficiency of insulin to promote glucose uptake in target tissues or to inhibit hepatic gluconeogenesis is decreased. In normal conditions, this insulin resistance is compensated by an increase of insulin release by the pancreas, thereby maintaining normal glucose tolerance [22, 23]. Type 2 diabetes mellitus is characterized by insulin resistance, which results in lower levels of blood glucose uptake into target tissues. Consequently, blood glucose levels increase and more insulin is released to compensate for the increase in blood glucose, producing hyperinsulinemia, which manifests early in type 2 diabetes. In addition, several studies have demonstrated that the hyper secretion of insulin is a primary defect of type II diabetes and that insulin resistance develops secondarily to the chronic hyperinsulinemia [24]. This study showed that a single dose of BPA resulted in hypoglycemia as observed in the BPA treated groups; however these rats presented hyperglycemia and insulin intolerance as shown in the AUC and plasma glucose values after 12 days of exposure to BPA. These alterations may have resulted from a direct effect of BPA on β-cell insulin content, which may have resulted in persistent hyperinsulinemia which subsequently led to alterations in glucose metabolism and insulin resistance. While BPA binds to the ERs and elicit an estrogen-like or even higher estrogen-like properties on the ERs [8, 25], studies have revealed that BPA also has an antagonistic effect on the AR [13, 12]. The anti-androgenic effect of BPA was reported by [13] in an in vitro study, they demonstrated that BPA blocked the action of dihydrotestosterone in a yeast screen containing a human androgen receptor; they reported that BPA was approximately as potent as flutamide, a well known anti-androgenic drug which binds and blocks the ARs [13]. Human studies also revealed that there is a correlation between BPA exposure and sexual dysfunction in men [26]. As shown in Fig. 4, the injection of high dose (4mg/kg) of BPA caused a significant (P<0.05) reduction in serum testosterone level in BPA treated groups compared with the control and low dose (100µg/kg) groups. Pre-treatment with either flutamide or tamoxifen did not block this effect. It is suggested that the high (4mg/kg) dose of BPA used in these groups may have elicited a higher anti-androgenic effect on the rats which may have overwhelmed the effects of pre-treatment with flutamide.
5. Conclusions
- In conclusion this present study therefore revealed that both androgen and estrogen receptors are important mediators of BPA’s effects in the alteration of glucose homeostasis and that BPA mediates its long term adverse effects on reproduction through both sex hormone receptors. Whatever the mechanism of action, BPA is an endocrine disruptor to which human exposure should be minimised.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML