-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2015; 4(1): 12-19
doi:10.5923/j.medicine.20150401.03
Effect of Different Conditions of Thyroid Function on Serum Adiponectin, Visfatin and Vaspin Levels in Rats
Mostafa H. Abdel Salam 1, 2, Husam M. Edrees 1, 3
1Department of Physiology, Faculty of Medicine, Zagazig University, Zagazig, Egypt
2Faculty of Medicine, Tabuk University, Saudi Arabia
3College of Public Health and Health Informatics, Qassim University, Saudi Arabia
Correspondence to: Husam M. Edrees , Department of Physiology, Faculty of Medicine, Zagazig University, Zagazig, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Adipocytokines are a variety of active biological substances secreted by adipose tissue. These substances have a pivotal role in control of metabolism, appetite, thermogenesis, thyroid and reproductive functions. Endocrine disorders were reported to affect their secretion and function. Abnormal levels of adipocytokines in hypo- and hyperthyroidism have been reported with controversial results. Therefore, this study was designed to demonstrate the effect of experimental hypo and hyperthyroidism on adiponectin, visfatin and vaspin levels in rats. Design: This study was performed on 45 adult male albino rats divided into 3 equal groups: Control (Group I), Hypothyroid (Group II) and Hyperthyroid (Group III) groups. Hypothyroidism was induced by propylthiouracil administration (0.05% in drinking water) for 3 weeks. Hyperthyroidism was inducted by L-thyroxine administration (250 µg/kg) subcutaneously for 2 weeks, while untreated animals served as controls. Results: The mean values for visfatin and vaspin were found to be significantly increased (P< 0.001) while Adiponectin was non-significantly changed in group II (Hypothyroid Group) when compared with group I (Euthyroid Group). In-group III (Hyperthyroid group), the mean values of adiponctin were found to be significantly increased (P< 0.001). While visfatin and vaspin were significantly decreased (P< 0.001) when compared with their respective values of group I and group II. Serum levels of adiponectin and visfatin were found to have significant positive correlation with serum levels of T3 and T4 and significant negative correlation with serum levels of TSH in all groups. However, serum levels of vaspin were found to have significant negative correlation with serum levels of T3 and T4 and significant positive correlation with serum levels of TSH in all groups. Conclusion: The results of this study suggest that adipokines can be affected by thyroid hormone disorders, so changes in the production of adipokines that accompany thyroid dysfunction may represent adaptive mechanisms to the changes in basal energy expenditure and in energy substrate requirements in thyroid disorders.
Keywords: Adipocytokines, Adiponectin, Visfatin, Vaspin, Hyperthyroidism, Hypothyroidism
Cite this paper: Mostafa H. Abdel Salam , Husam M. Edrees , Effect of Different Conditions of Thyroid Function on Serum Adiponectin, Visfatin and Vaspin Levels in Rats, Basic Sciences of Medicine , Vol. 4 No. 1, 2015, pp. 12-19. doi: 10.5923/j.medicine.20150401.03.
Article Outline
1. Introduction
- Adipose tissue is a complex organ including adipocytes, immune cells, fibroblasts, blood vessels, and collagen fibers. It is classified as brown adipose tissue (BAT) and white adipose tissue (WAT). WAT is the predominant type because BAT regresses after birth. Leptin, adiponectin (ADP), vaspin, visfatin, interleukin 6 (IL6), plasminogen activator inhibitor type 1, and resistin are among the adipocytokines secreted from the WAT [1].Thyroid hormones are mainly involved in the regulation of the metabolic rate and energy metabolism [2]. They can stimulate uncoupling of oxidative phosphorylation, enhance the responsiveness of catecholamines and modulate the number of their receptors [2-4]. Moreover, Thyroid-stimulating hormone (TSH) receptors have been found in the adipose tissue; this indicates that it can perform a central role in the regulation of adipose tissue metabolism [5]. Changes of thyroid hormones in patients with thyroid dysfunction have been previously shown to alter adipocytokines secretion [6]. Experimental studies also revealed that triiodothyronine (T3) could accelerate adipocyte differentiation [7, 8].Adiponectin is a 30 KDa protein abundantly secreted from adipocytes and circulates at high concentration in the blood as three oligomeric complexes [9, 10]. Two different types of ADP receptors (AdipoR), 1 and 2, have been identified [11]. AdipoR1 is expressed primarily in muscles and AdipoR2 is expressed primarily in liver. [12]. Given that thyroid hormones share some physiological actions with ADP (i.e. such as reduction of body fat by increased thermogenesis and lipid oxidation) [13], it is conceivable that ADP may interact with thyroid axis. However, studies have revealed contradicting reports as regard the effect of different thyroid conditions on Adiponectin.Hypothyroid rats have either increased or unchanged serum ADP levels, while unchanged or increased serum ADP levels are found in hyperthyroid rats [14, 15].Visfatin is one of the most abundant adipocytokines recently discovered with the capacity to modulate several functions. Visfatin receive much attention as it was reported to function as an insulin sensitizer [16]. Plasma visfatin levels are highly correlated to the amount of visceral fat [17, 18]. Controversial results are reported concerning the role of thyroid hormones in the regulation of visfatin, as it was reported that hyperthyroid patients had significantly lower visfatin levels compared with the hypothyroid group and controls. Plasma visfatin level decreased significantly after treatment in the hypothyroid group, whereas it increased significantly after treatment in the hyperthyroid group [19]. In contrast, another study reported elevated plasma Visfatin concentrations in both hypo- and hyperthyroid groups compared with control group [20].Visceral adipose tissue (VAT) carries a greater metabolic risk than subcutaneous adipose tissue [1]. Hida et al. [21] identified a novel insulin-sensitizing adipocytokine called VAT-derived serine protease inhibitor (vaspin) from the VAT of obese, diabetic rats. Vaspin is a member of the serine protease-inhibitor family (serpins). They reported that administration of vaspin to obese, diabetic rats caused improvement in glucose tolerance and insulin sensitivity). In addition, other studies have documented positive association between vaspin gene expression in human adipose tissue and serum vaspin levels with obesity and type 2 diabetes [22, 23].Gonzalez et al. [24] showed that vaspin mRNA levels are significantly decreased in hyperthyroid rats and significantly increased in hypothyroid rats compared with the euthyroid ones. Despite there being no change in glucose and insulin levels, suggesting that thyroid dysfunction may affect vaspin expression. Handisurya et al. [25] reported a significant positive correlation between serum TSH and serum vaspin levels. In contrast, Vaspin levels were found to be similar in euthyroid and hypothyroid subjects, and no significant difference was observed. Moreover, vaspin levels were not correlated with TSH [26].The pathophysiological role of thyroid hormones in the regulation of ADP, vaspin, and visfatin is still unclear. Changes in adipokine secretion with thyroid dysfunction may represent adaptive mechanisms to the decrease or increase in basal energy expenditure and in energy substrate requirements in thyroid dysfunction. Therefore, this study was designed to evaluate the effect of experimental thyroid dysfunctions in rats on Adiponectin, Visfatin and Vaspin levels.
2. Material and Methods
2.1. Experimental Animals
- The current study was carried on 45 adult male albino rats 16 – 20 weeks old with body weight 190-255 gm, were obtained from the animal house from faculty of veterinary medicine of Zagazig University. Rats were kept in steel wire cages (10/cage) in the physiology research laboratory in faculty of medicine of Zagazig University under hygienic conditions, rats were kept on the normal diet, which consisted of mixed commercial rat laboratory chow and supplied in separate clean containers. Animals had free access to water, kept at room temperature and were maintained on a 12 hr light/dark cycle. All the animals were breed in the animal house and they fed the same type of food to avoid the effect of different food elements on the experiments [27].
2.2. Methods
- The rats were accommodated to laboratory conditions for two weeks before the beginning of experiments. Rats were numbered and divided into the following groups:Group I: (Control group- n = 15): In which the rats received no treatment.Group II: (Hypothyroid Group- n = 15): In which the rats were subjected to propylthiouracil (0.05%) in drinking water for 21 days [14].Group III: (Hyperthyroid Group- n = 15): In which the rats were administered with L-thyroxine (250 μg/kg) SC, once daily, for 14 days [14].
2.3. Drugs and Chemicals
- Plasma L-thyroxine and 3, 5,3 tri-iodothyronine quantitative Measurements were performed with ELISA, using kits obtained from Alpha Diagnostic International, Texas, USA (No. 1100 for total T4 and No. 1700 for total T3). Absorbance measurements were performed as described by Pantos et al. [28].1. L-thyroxin: Sigma Chemicals, St. Louis MO, USA.2. Propylthiouracil: Sigma Chemicals, St. Louis MO, USA.2.2.3.3. Kits for Adiponectin level estimation: ADIPOQ / Adiponectin EIA Kit/Sigma-Aldrich Co. USA.4. Kits for Visfatin level estimation: Visfatin EIA Kit/Sigma-Aldrich Co. USA.5. Kits for T3, T4 and TSH: Siemens healthcare Diagnostics, Deerfield, IL).
2.4. Sampling of Blood
- At the end of the study protocol, all animals were anaesthetized, sacrificed by decapitation and blood was obtained, centrifuged at 7000 rpm. The serum was separated then stored at -20 c in a dark container until measurement of T3, T4, TSH, Adiponectin, Visfatin and vaspin levels.
2.5. Statistical Analysis
- Data are shown as the mean ± SEM. Shapiro-Wilk and Levene median tests were performed to assess normality and equal variance, respectively. If p > 0.05 was reached for both tests, (i.e. Normal distribution of data) one way Analysis of variance (ANOVA) was performed followed LSD Post-Hoc test for multiple comparisons. If p < 0.05 (i.e. Non-normally distributed data) Kruskal Wallis test was used instead for the comparison of data between groups. P values less than 0.05 (p<0.05) were considered significant. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS), version 17.0 (SPSS Inc., Chicago, IL, United States).
3. Results
- Table 1 and 2 and histogram 1 and 2 show serum level of T3, T4 and TSH in all studied groups expressed as mean ± standard deviation. In group II (Hypothyroid Group), the mean values were found to be significantly decreased (P< 0.001) for serum levels of T3 and T4 and significantly increased (P< 0.001) for serum levels of TSH, visfatin and vaspin however, the mean values were found to be non-significantly changed for serum levels of Adiponectin when compared with that of group I (Euthyroid Group).
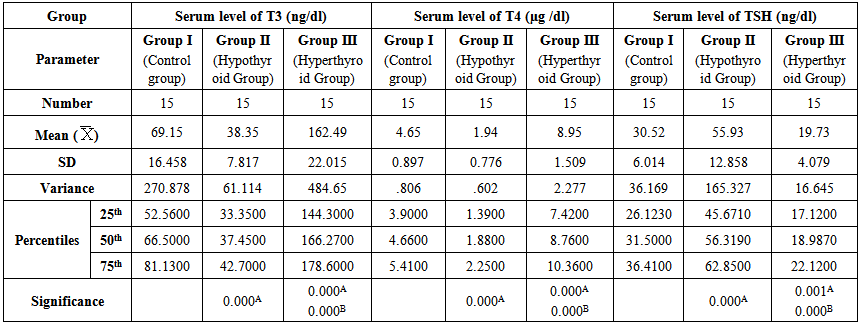 | Table 1. Serum levels of T3, T4 and TSH in different groups |
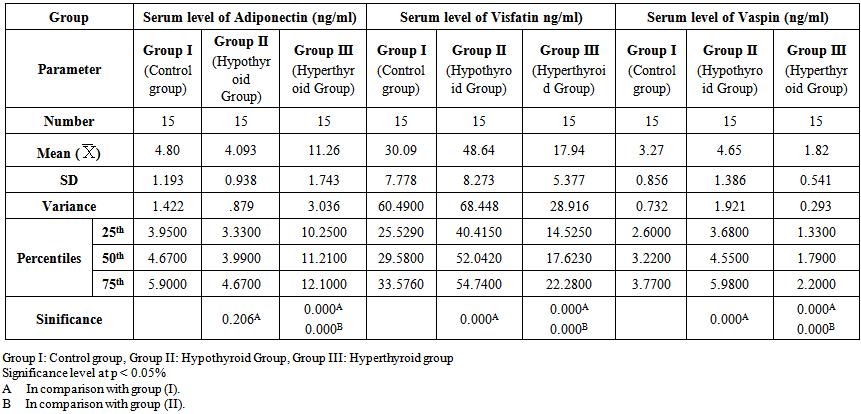 | Table 2. Serum levels of adiponectin, visfatin and vaspin in different groups |
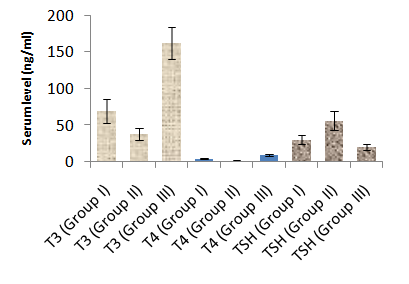 | Histogram 1. Serum levels of T3, T4 and TSH in different groups |
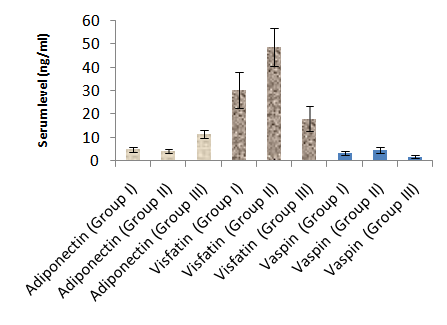 | Histogram 2. Serum levels of Adiponectin, Visfatin and Vaspinin different groups |
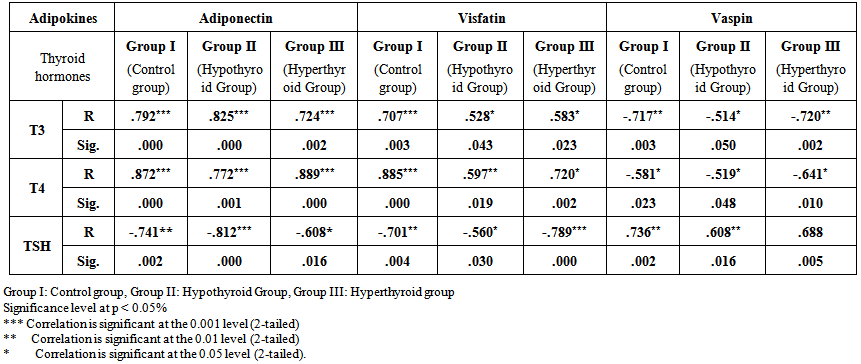 | Table 3. Correlation between Serum levels of adiponectin, visfatin and vaspin and thyroid hormones levels in different groups |
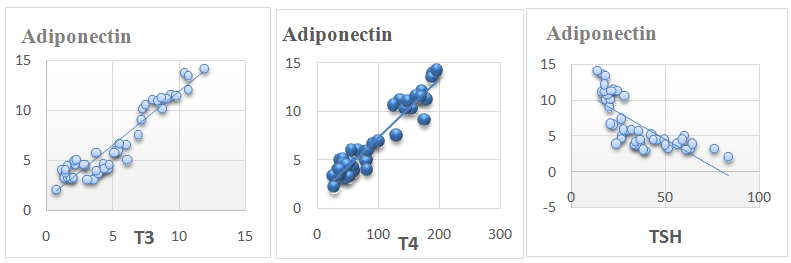 | Figure 1. Showing correlation of serum adiponectin vs. serum T3, T4 and TSH levels in all animals |
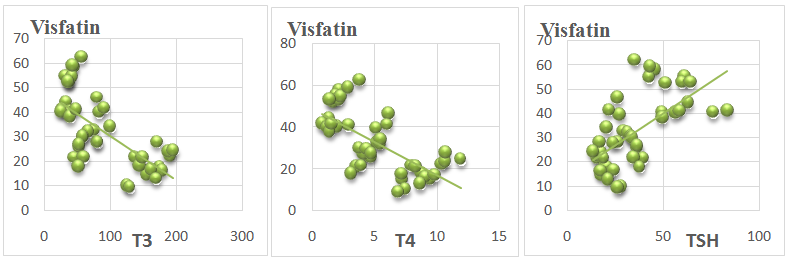 | Figure 2. Showing correlation of serum visfatin vs. serum T3, T4 and TSH levels in all animals |
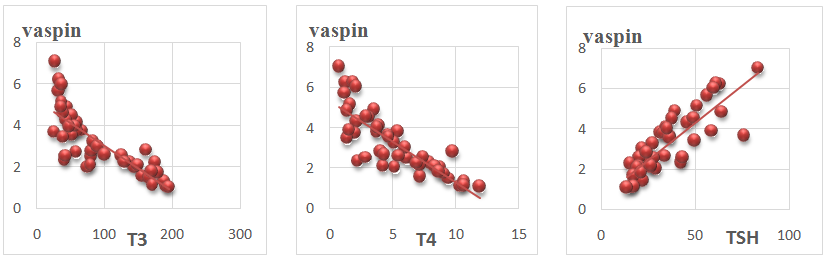 | Figure 3. Showing correlation of serum vaspin vs. serum T3, T4 and TSH levels in all animals |
4. Discussion
- In this study, it was found that hypothyroidism (Group II) did not significantly affect serum adiponectin level when compared with control group (group I). While, hyperthyroidism (group III) induced a significant increase in serum adiponectin level when compared with hypothyroid and control groups. Moreover, Serum adiponectin level showed a significant positive correlation with serum T3 and T4 Levels and a significant negative correlation with serum TSH levels in all study groups.These results are in agreement with Aragao et al. [15] who reported that Serum adiponectin level of hyperthyroid rats was significantly higher than that of euthyroid ones, whereas that in hypothyroid rats tended to be lower, but without statistical significance. They reported also, that serum adiponectin had a significant positive correlation with serum thyroxine (T4) and triiodothyronine (T3) and a significant negative correlation with serum thyroid-stimulating hormone (TSH). In contrast, Kokkinos et al. [14] reported that adiponectin level was significantly increased in hypothyroid rats as compared to control rats. Moreover, Seifi et al. [29] reported that adipose tissue adiponectin mRNA was significantly elevated in cases of hyperthyroidism agreeing with this study. However, In contrast to our study, they reported a significant decrease in adiponectin mRNA in cases of hypothyroidism.The higher adiponectin level can be related to the changes in visceral fat presented in hyperthyroid rats [15]. Moreover, there is a possibility of a direct T4 action on adiponectin expression. The effect of T4 on adiponectin production from primary white adipocytes is unknown, although in the adipocyte cell line 3T3-L1 [30]. T3 was not able to modify adiponectin messenger RNA expression. However, in brown adipocyte cultures, incubation with T4 resulted in a modest increase in the secretion and expression of adiponectin [31]. It is interesting to note that BAT is enlarged in hyperthyroid rats and there was a positive correlation between BAT mass and serum adiponectin level. Thus, it raises the possibility that BAT may be one contributing source of adiponectin in hyperthyroid rats [15].Moreover, Cabanelas et al. [32] showed that T3 administration in rats had no significant effect on adiponectin (ADP) secretion in visceral (epididymal) and subcutaneous (inguinal) adipose tissues, while ADP mRNA expression was down regulated by T3 in the subcutaneous adipose tissue, but not in the visceral adipose tissue (VAT).In this study, it was found that hypothyroidism (Group II) significantly increased serum visfatin level when compared with control group (group I). While, hyperthyroidism (group III) induced a significant reduction in serum visfatin level compared with hypothyroid and control groups. Moreover, Serum visfatin level showed a significant negative correlation with serum T3 and T4 Levels and a significant positive correlation with serum TSH levels in all study groups.These results are in agreement with Ozkaya et al. [33] who found markedly low visfatin levels in hyperthyroid patients compared with the hypothyroid and control groups. They observed also a significant positive correlation between visfatin and TSH levels and a significant negative correlation between visfatin levels and T3 and T4 values. This could be explained by the findings of McLaren et al. [34] who reported that T3 could induce a down-regulation of visfatin mRNA expression in 3T3-L1adipocytes. In contrast Caixas et al. and [35] reported that serum visfatin levels is significantly elevated in both hypo and hyperthyroid groups compared with controls, and that it even further increased after treatment of both thyroid disorders. They also, showed no significant correlations between visfatin and any other parameters. Similar results were detected by Han et al. [20]; however, the same authors reported that high concentrations of T3 might exert a lipolysis-dominant effect on adipocytes, resulting in the reduction of visfatin, which is similar to our results.In this study, it was found that serum vaspin levels were significantly increased in hypothyroidism and decreased in hyperthyroidism as compared to control group. Moreover, serum vaspin level showed a significant negative correlation with serum T3 and T4 Levels and a significant positive correlation with serum TSH levels in all study groups.These results are in agreement with Gonzalez et al. [24] who reported that Vaspin mRNA levels were significantly down regulated in hyperthyroid rats and significantly increased in hypothyroid rats with respect to euthyroid rats. They indicated that white adipose tissue (WAT) vaspin mRNA expression was affected by thyroid status. Moreover, another study reported a significant positive correlation between serum TSH and serum vaspin levels [25]. This decrease of vaspin production may be due to the lipolysis-dominant effect of T3 on adipocytes [20]. In contrast, in a clinical study in humans, Cinar et al. [26] reported that vaspin levels were neither altered in manifest and subclinical hypothyroidism as compared to control group. They also reported that vaspin level was not correlated with TSH levels indicating that thyroid hormones do not affect vaspin production. This difference may be due to different effects of thyroid hormones in rats and humans.
5. Conclusions
- In conclusion, this study shows that adipokines can be affected by thyroid hormone disorders where hypothyroidism induced a significant increase in visfatin and vaspin levels with no effect on adiponectin. In addition, hyperthyroidism induced a significant decrease in visfatin and vaspin levels. However, the pathophysiological role of these changes is still unclear. Changes in the production of adipokines that accompany thyroid dysfunction may represent adaptive mechanisms to the changes in basal energy expenditure and in energy substrate requirements in thyroid disorders.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML