-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2014; 3(4): 63-68
doi:10.5923/j.medicine.20140304.01
Serum Kisspeptin Level in Experimentally Induced Hypogonadism Model of Adult Female Rats and Its Effect on Fertility
Rania R. A. Atia, Khaled A. A. Abulfadle
Physiology department, Faculty of Medicine, Zagazig University, Egypt
Correspondence to: Rania R. A. Atia, Physiology department, Faculty of Medicine, Zagazig University, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
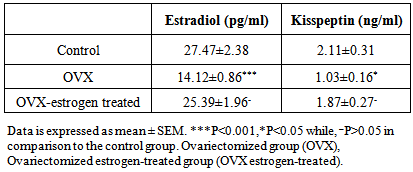
Objective:To investigate the effect of experimentally induced hypogonadism model of adult female rats on serum kisspeptin level and its effect on fertility.Experimental design: Total number of 18 healthy, adult, female albino rats were used. Rats were divided into three groups 6 rats each. Group I (control). Group II (ovariectomized, OVX). Group III (OVX estrogen-treated)were bilaterally ovariectomized adult female rats with recovery for 21 days then, three s.c. daily injections with estradiol (E2) penzoate (2.5 μg/kg body weight) dissolved in sesame oil for three days. In all the three groups, kisspeptin level and estradiol levels were estimated at the proper time for each group.Results: This study revealed that there is a significant decrease in serum levels of both estradiol (14.12±0.86) and kisspeptin (1.03±0.16) in ovariectomized (OVX) group in comparison to those in the control group (27.47±2.38) and (2.11±0.31) respectively. But, there is an insignificant decrease in serum levels of both estradiol (25.39±1.96) and kisspeptin (1.87±0.27) in OVX estrogen-treated group in comparison to those in the control group (27.47±2.38) and (2.11±0.31) respectively.Conclusions: There is a link between serum levels of kisspeptin and estradiol which confirmed that sex steroids play a major role in serum kisspeptin level regulation which in turn affects fertility. To our knowledge, this is the first study to estimate the effect of ovariectomy and estradiol replacement on serum levels of kisspeptin in experimental animal models.
Keywords: Kisspeptin, Estradiol, Fertility, Ovariectomy
Cite this paper: Rania R. A. Atia, Khaled A. A. Abulfadle, Serum Kisspeptin Level in Experimentally Induced Hypogonadism Model of Adult Female Rats and Its Effect on Fertility, Basic Sciences of Medicine , Vol. 3 No. 4, 2014, pp. 63-68. doi: 10.5923/j.medicine.20140304.01.
Article Outline
1. Introduction
- Metastin, a metastasis suppressor gene encoding a 54-amino-acid peptide is known asKiSS-1. [1] KiSS-1 gene gives origin to kisspeptins by differential proteolytic processing of its product. [2] Actions of kisspeptins are conducted through G protein-coupled receptor 54(GPR54). [3] KiSS-1/GPR54 system has been involved in tumor progression and metastasis, and potent antimetastasis actions of KiSS-1 peptide have been described in papillary thyroid carcinoma, breast carcinoma, melanoma, and pancreatic cancer cells. [4] In addition, it was recently proposed that KiSS-1 peptides likely play a role in the physiological regulation of trophoblast invasion [5] and initial evidence suggested that KiSS-1 may participate in the regulation of specific neuroendocrine systems asthe release of oxytocin. [2] Indeed, the expression of KiSS-1/GPR54 system has been found in placenta, different brain areas as hypothalamus, spinal cord, pituitary, pancreas, and human plasma [6], which strongly suggests additional physiological functions of this newly discovered system. Recently, it was found that there is a role for KiSS-1/GPR54 system in the neuroendocrine control of gonadotropin secretion, brain sex differentiation, puberty onset and fertility [7]. In addition, studies on rodents and sheep suggested that hypothalamic expression of Kiss1 and/or kisspeptin fiber distribution are altered after inappropriate exposures to synthetic estrogenic compounds during development. [8] On the other hand, mice and humans lacking kisspeptin receptor expression showed a phenotype of hypogonadotrophic hypogonadism and consequent infertility. [9] Moreover, Kiss1 system has been recently recognized as a regulator of the reproductive axis, which is indispensable for its timely activation at puberty and its proper function including fertility during adult life. [10] The primary mechanism whereby kisspeptins participate in the control of the reproductive axis involves its ability to operate upon, and stimulate, GnRH neurons, which have been shown to express GPR54. [11]. It was found that blocking of kisspeptin actions causes elimination of the pre-ovulatory peak of gonadotropins in cyclic female rats. [12] Also, kisspeptins play an essential role in regulation of timing of puberty, as evidenced by a combination of genetic, physiologic and pharmacological analyses in rodents and primates. [13] The hypothalamic Kiss1 system undergoes a complex activation program during puberty, which involves elevation in the endogenous kisspeptin tone at the hypothalamus [14], increase in the sensitivity to the stimulatory effects of kisspeptins in terms of GnRH/LH responses [15], enhancement of GPR54 expression and signaling efficiency [16], and elevation of the number of kisspeptin neurons and of their projections to GnRH neurons. [17] Estradiol was recognized as an important regulator of hypothalamic Kiss1 gene expression during adulthood as evidenced by experiments of gonadectomy, with or without sex steroid replacement in rodents. [10] A similar regulatory action is operative also in humans, as demonstrated by studies on the changes in hypothalamic Kiss1 mRNA levels in postmenopausal women. [18] It was found that neonatal estrogenization of female rats decreased the number of hypothalamic Kiss1 neurons and disrupted the positive feedback effects of estradiol on LH secretion in adulthood. [19] Also, neonatal exposures to synthetic estrogens, disturb proper activation and function of the gonadotropic axis, persistently suppressed the hypothalamic expression of Kiss1 gene at the expected time of puberty and adulthood in male and female rats. [20] Furthermore, neonatal exposure to estrogens decreased kisspeptin fiber density at certain hypothalamic nuclei in adult female rats and caused also reduced GnRH neuronal activation. [21] Although the role of kisspeptin in the reproductive system and puberty is well established, yet, participation of a kisspeptin ergic system in follicular development remains unexplored. The delay in vaginal opening as an index of puberty in the rat after the direct ovarian administration of a kisspeptin antagonist from prepuberty to adulthood clearly demonstrates that the ovary depends on kisspeptin to initiate a normal reproductive cycle. This was experimentally supported by the marked irregularities in the estrous cycling activity of the rats treated with intraovarian kisspeptin antagonist [22]. Some studies found a close association between decrease in the plasma levels of kisspeptin and estradiol and the changes in estrous cyclingactivity. [22] This study was designed to investigate the effect of gonadectomy and estradiol replacement on serum kisspeptin levels in animal model.
2. Materials and Methods
2.1. Animals
- 18adult (2-6 months; body weight, 180-250 gm) healthy female albino rats were obtained. From the animal house from Faculty of Medicine Zagazig University. Animals were kept in steel wire cages (4/cage) in the physiology research laboratory department in faculty of medicine Zagazig University. Under hygienic conditions rats were kept on the diet, which consisted of mixed commercial rat laboratory chow and supplied in separate clean containers. Animals had free access to water, kept at room temperature and were maintained on a 12 hour light/dark cycle. All the animals were bred in the animal house and they fed the same type of food to avoid the effect of different food elements on the experiments. [21]The rats were accommodated to laboratory conditions for two weeks before the experiments going on.
2.2. Chemicals
- Kisspeptin commercial enzyme immunoassay (EIA) kit (Phoenix Pharmaceuticals Inc., Burlingame, California, USA). Estradiol benzoate in an oily solution (Misr CO. pharm. Ind. S.A.A. Materia. Cairo-A.R.E. C.C.R. 32048). Estradiol (E2) Enzyme Immunoassay Test Kit: Catalog Number: 11110 (Oxis International, Inc323 Vintage Park Dr. Foster City, CA 94404). Sesame oil from sesamum indium Ph. Eur. obtained by expression (Fluka chemie. Gm. bH, CH-9471 Buchs, Sigma-Aldrich chemie Gm bH, Ried Str. 2, D-89555 Steinheim 07329/970. Packed in Switzerland).
2.3. Methods
- Rats were divided into three groups, 6 rats each. Rats of group I (control) were kept with intact gonads and used as control. Rats of group II (ovariectomized, OVX) were bilaterally ovariectomized adult female rats with recovery for 21 days. Rats of group III (ovariectomized estrogen-treated) were bilaterally ovariectomized adult female rats with recovery after 21 days then three subcutaneous (s.c.) daily injections with estradiol (E2) penzoate (2.5 μg/kg body weight) dissolved in sesame oil (used as a vehicle for fat soluble hormones because of its high stability)for three days. All groups were maintained for 21 days and the injected group was supplemented for 3 days. The blood samples were obtained 12 h after the last dose of hormonal replacement. [23] In all three groups, estradiol and kisspeptin levels were measured by commercial kits. Adult female rats at the beginning of the experiment were monitored for estrous cycle by daily vaginal cytology; only rats with at least two consecutive regular4-day estrous cycles were used for subsequent studies.
2.4. Ovariectomy Technique
- Rats were fasted overnight, they were anaesthetized by intraperitoneal anesthesia with pentobarbital sodium (40 mg/kg body weight), and then the anaesthetized rat were tied to the operating board. [24] The hair was removed from the lower abdomen by small curved scissor. The bare area of the skin was sterilized by 70% of ethyl alcohol. Then, a midline incision was made through the skin and the abdominal wall, after that the ovaries were explored. For ovariectomy, after abdominal incision, the uterus, oviducts, and ovaries were exposed with the fat tissues around them, the oviduct and blood vessels supplying the ovaries were tied up, and the ovaries were removed through an incision and removed surgically. The viscera were replaced and the abdominal wall was repaired with catgut thread, then, the skin incision was closed with sterile silk suture. At the end, garamycin cream was put over the closed incision and covered with a sterilized gauze, and then the rats were observed until the recovery from anesthesia. The procedure was identical during sham OVX excluding clamping the fallopian tubes and removal of the ovaries. [25] General hormonal status was assessed throughout the study by daily vaginal smears obtained ~2 hours after light onset. [25] Only rats whose vaginal smears indicated that the rat exhibited constant leukocytes (after OVX), and that the rat exhibited constant cornified cells (after OVX-estrogen-treated) were used.
2.5. Sampling of Blood
- Upon decapitation, trunk blood was collected. The blood samples were allowed to clot for 2 hours at room temperature before centrifuging for 20 minutes at approximately 2000 x g. The serum was separated and stored at -20°C until use for hormone determinations. Repeated freezing was avoided. [7]
2.6. Analysis of Serum Kisspeptin Level
- Serum kisspeptin levels were measured by a commercial enzyme immunoassay (EIA) kit (Phoenix Pharmaceuticals Inc., Burlingame, California, USA). The range of kisspeptin was 0-100 and the minimum detectable concentrations were 0.06 ng/ml. The absorbance O.D. was read at 450 nm by Synergy HT Multi-Detection Microplate Readers (Bio Tek Instument, Inc., Winooski, VT, U.S.).
2.7. Statistical Analysis
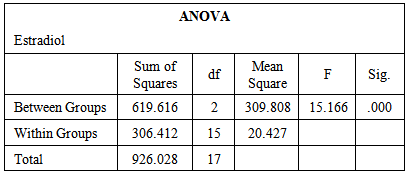
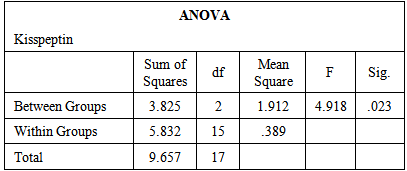
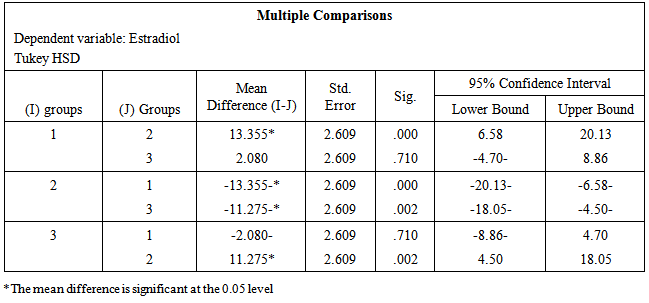
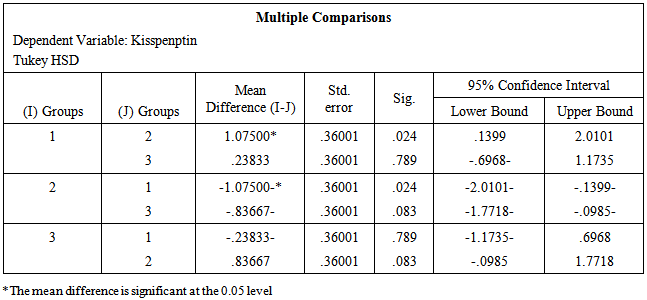
- The data obtained from serological examination of estradiol and kisspeptin was expressed as mean values ± standard error of the mean (SEM). Means were compared by one-way ANOVA and Tukey HSD for Post Hoc Multiple Comparisons using (IBM SPSS Statistics Version 21 Software for Windows) for statistical significance. P value of 0.05 or less was taken to indicate statistical significance.
3. Result
- Table-1 and figure-1 showed that there is a significant decrease in serum levels of both estradiol (14.12±0.86) and kisspeptin (1.03±0.16) in ovariectomized (OVX) group in comparison to those in the control group (27.47±2.38) and (2.11±0.31) respectively. But, there is an insignificant decrease in serum levels of both estradiol (25.39±1.96) and kisspeptin (1.87±0.27) in OVX estrogen-treated group in comparison to those in the control group (27.47±2.38) and (2.11±0.31) respectively.
|
|
|
|
|
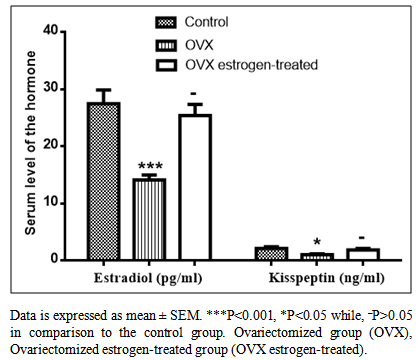 | Figure 1. Serum levels of estradiol (pg/ml) and kisspeptin (ng/ml) in control, ovariectomized (OVX) and the OVX estrogen-treated groups |
4. Discussion
- The results of our study confirmed that estradiol is a regulator for serum level of kisspeptin, as there is a significant decrease in serum level of both kisspeptin and estradiol in the ovariectomized group in comparison with the control one, but, there is an insignificant change in their serum levels in ovariectomized group treated with estradiol in comparison with those of the control group. This confirms that the ovaries secret kisspeptin or at least its hormone, estradiol, affects kisspeptin secretion. This is supported by Iwasa et al. [26] who stated that, in peripheral tissue, cells expressing the KISS1 gene include pituitary, syncytiotrophoblast and ovary in humans and other species. This is also supported by, d’Anglemont et al. [27] who found that genetic inactivation of GPR54 andKiSS-1 has been reported to cause sexual immaturity and hypogonadotropic hypogonadism. Moreover, the results of our study are supported by Horikoshi et al. [6] who estimated high levels of kisspeptin in the peripheral blood of pregnant women. This is further supported by Smith et al. [28] who stated that, gonadectomy decreased Kiss1 expression in the AVPV and sex steroid replacement restored it. Indeed, Adachi et al. [29] reported that administration of kisspeptin-blocking antibodies to the brains of female rats blocks the mid-cycleLH surge. On the other hand, Smith et al. [28] found that gonadectomy increases the number of detectable Kiss1 mRNA expressing neurons as well as the content of Kiss1 mRNA per cell in the Arc and sex steroid replace mentreduces Kiss1 expression back to that of intact animals and they suggested that kisspeptin may modulate the negative feedback on GnRH secretion exerted by sex steroids. Therefore, sexsteroids appear to play a major role in kisspeptin expression. Also, the KiSS-1/GPR54 system has been implicated in the dynamic regulation of gonadotropin secretion in adulthood, playing key roles in conveying both positive and negative feedback effects of sex steroids and the metabolic control of the gonadotropic axis and its modulation by endogenous rhythms and environmental cues. [30] In mice and monkeys, Kiss1 mRNA levels in the hypothalamus are low prior to sexual maturation but increase dramatically at the time of sexual development. [14] Both male and female rats undergo considerable augmentation of hypothalamic Kiss1 mRNA expression during the transition from juvenile to adult life. [31] More specifically, Kiss1 expression in the mouse AVPV (both number of neurons and kisspeptin contentper cell) is greater in adult than in prepubertal animals. [32] In addition to increase in kisspeptin expression, kisspeptin tone appears to play a pivotal role in the onset and pacing of reproductive development. In sexually immature female rats, chronic administration of kisspeptin (6 days) advances the timing of sexualmaturation as evidenced by precocious vaginal opening. [31] In the human, mutations with in the kisspeptin/Kiss1R pathway established varying degrees of kisspeptin tone with clear effects on the timing and pace of pubertal development. As mentioned earlier, loss-of-function mutations in the gene encodingKiss1R cause hypogonadotropic hypogonadism. Recently, a gain-of-function mutation inKiss1R has been identified in a patient with central precocious puberty. [33] Thus, kisspeptin is an indisputable gatekeeper of pubertal function and fertility. Further work should be done to evaluate the effect of kisspeptin on fertility and how it may be a line of infertility treatment.
5. Conclusions
- There is a link between serum levels of kisspeptin and estradiol which confirmed that sexsteroids play a major role in serum kisspeptin level regulation which in turn affects fertility. To our knowledge, this is the first study to estimate the effect of ovariectomy and estradiol replacement on serum levels of kisspeptin in experimental animal models.
ACKNOWLEDGEMENTS
- We provide our sincere gratitude to Physiology department, Faculty of Medicine, Zagazig University, Egypt, for supporting this study to be achieved.
Conflict of Interest
- The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the article reported.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML