-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2014; 3(3): 43-59
doi:10.5923/j.medicine.20140303.02
Effect of Obestatin on Gonadal Functions in High Fat-fed Albino Rats
Mohmmad H. M. Ibrahim, Soad A. A. Selim, Dalia I. Abd-Alaleem, Radwa M. Al-sayed
Physiology department, Faculty of Medicine, Zagazig University
Correspondence to: Dalia I. Abd-Alaleem, Physiology department, Faculty of Medicine, Zagazig University.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Background:Obestatin is a peptide potentially produced in the stomach, pancreas and testis. Recently, obestatin was reported as a novel adipocytokine and its levels were decreased in obese subjects.Objective: This study was designed to explore the probable effects of obestain peptide in modulating the adverse effects of obesity on gonadal functions with a trial to clarify some of the possible underlying mechanisms.Animals and methods: 64 wistar albino rats 21-day old (32 males and 32 females) were divided equally (8males and 8females) into 4 groups, group (Ia): Saline-vehicle treated normal fed group, group (Ib): Rats were fed a normal chow for 10 weeks then obestatin was i.p injected (1 nmol) daily for 10 days, group (IIa):Saline-vehicle treated high fat fed group and group (IIb): Rats were fed high fat diet for 10 weeks then injected i.p with obestatin (1 nmol) daily for 10 days. 5 days after the end of obestatin administration BMI and AC/TC ratio were calculated, serum glucose& insulin (with calculation of HOMA-IR), lipid profile, FSH, LH, estradiol, in addition to testosterone (in male rats), and progesterone (in female rats), were estimated, mesenteric fat, epididymal fat, periovarian fat, the right testes and ovaries, were weighed. The epididymes were used for the evaluation of sperm parameters. The testes and ovaries were processed for histopathological studies.Results:There was a significant (p<0.001) increase in the anthropometric parameters together with deterioration of metabolic and gonadal functions in high fat fed group (group IIa), while exogenous obestatin administration in (group IIb) resulted in: Significant decrease in the anthropometric parameters and the fat weight, significant decrease in insulin resistance (HOMA-IR) (p<0.001), significant increase in serum LH (p<0.001)&testosterone (p<0.01), sperm count (p<0.01) and motility (p<0.001) in male rats. Moreover, there was a significant increase in serum LH (p<0.05) & progesterone (p<0.05) and restoration of the regularity of the estrus cycle (p<0.0001) in female rats. Together with marked improvement in the gonadal histoarchitecture. Conclusions: It could be suggested that obestatin has a potential positive role against obesity-induced gonadal dysfunction, which may be due to its role in maintenance of glucose & insulin homeostasis, and/ or maintenance of gonadal hormonal function via indirect and/or direct effect on the gonad.
Keywords: Obestatin, Obesity, Testicular function, Ovarian function
Cite this paper: Mohmmad H. M. Ibrahim, Soad A. A. Selim, Dalia I. Abd-Alaleem, Radwa M. Al-sayed, Effect of Obestatin on Gonadal Functions in High Fat-fed Albino Rats, Basic Sciences of Medicine , Vol. 3 No. 3, 2014, pp. 43-59. doi: 10.5923/j.medicine.20140303.02.
Article Outline
1. Introduction
- Obesity which is a worldwide health problem is reported to be a major susceptibility factor leading to the development of various conditions of the metabolic syndrome [1, 2].Obesity also lead to disruptions in reproduction both in male and female, as obesity has been reported to affect fertility in males by decreasing the quantity of spermatozoids [3-5]. Furthermore, it has been seen that obese males frequently show a low hormonal profile for testosterone and a high profile for estradiol [6, 7] and this pattern is proportional to the degree of obesity [8-10]. In humans, obesity in females reduces pregnancy rates, increases complications with polycystic ovarian syndrome, and induces annovulatory cycles and irregular menses [11]. However, the mechanisms by which excess body fat interferes with reproductive functions are still not fully understood, especially if the increased intake of high-calorie food is occurring at earlier ages, considering that alterations on reproductive parameters are directly proportional to the duration of the diet [12, 13].Obestatin is 23-amino acid peptide hormone generated from proteolytic cleavage of preproghrelin, that is potentially produced in cells of the gastric mucosa, myenteric plexus, pancreas and in Leydig cells of the testis [14-16]. It has been reported to bind to and activate the orphan receptor, G protein-coupled receptor-39 (GPR39) [14].Obestatin seems to function as part of a complex gut–brain network where hormones and substances from the stomach and intestine signal the brain about satiety or hunger [17]. In contrast to ghrelin, which causes hyperphagia and obesity in rats [18], obestatin appears to act as an anorexic hormone, decreasing food intake, slowing gastric emptying and jejunal motility, and reducing body weight gain in rodents [19].Furthermore,serum levels of this hormone has been found to correlate with obesity measures such as body mass index, and waist circumference, as well as with insulin concentration and insulin resistance [20-22]. In addition, Qi et al. [23] and Abou-Fard et al. [24] studies showed a decreasing circulating levels of obestatin in type II diabetic and obese rats. Data regarding the involvement of obestatin in the reproductive functions is still lacking, however, it was found that obestatin significantly increased progesterone secretion in cultured porcine ovarian granulosa cells [25]. Moreover, in adult male rats, it was reported that obestatin can induce testosterone secretion [26]. Therefore, this study was designed to explore the probable effects of obestain in modulating the adverse effects of obesity on gonadal functions with a trial to clarify some of the possible involved mechanisms.
2. Animals and Methods
- A total number of 64 wistar albino rats 21-day old (32 males and 32 females) weighing 80-100 g were obtained from the animal house of Faculty of Veterinary Medicine-Zagazig University. The animals were kept in steel wire cages (8 rats per cage) and females were separated from males to avoid conception.They were housed in an air-conditioned room with controlled lighting (12 hours light/12 hours dark cycle) and temperature (21-24°C) and received food and water ad libitum.The animals were randomized into 2 equal groups: Group (I): normal fed group consisted of 32 rats (16 male and 16 female rats) in which rats were fed normal diet for 10 weeks then, further divided into 2 equal subgroups (8males, 8females); Group (Ia)- Saline vehicle treated group, each rat received 100 μl of saline (i.p) daily in the morning for 10 consecutive days. Group (Ib)- Obestatin treated group, each rat received 1 nmol obestatin (acylated powder form, Sigma Aldrich Co.-USA) /100 μl saline (i.p) daily in the morning for 10 consecutive days [26]. Group (II): High-fat fed group: in which rats were fed high fat diet for 10 weeks [27] then, further divided into 2 equal subgroups(8males, 8females): Group (IIa)- Saline vehicle treated high-fat fed (HFF) group, each rat received 100 μl saline (i.p.) daily in the morning for 10 consecutive days. Group (IIb)- Obestatin treated high fat fed group, each rat received 1 nmol obestatin/100 μl saline (i.p) daily in the morning for 10 consecutive days [26]. Rats in normal fed groups received standard chow 3.89 Kcal/gm (Casein 33.11%, Cystine 0.30%, Starch 25.21%, Dextrose 25.21%, Cellulose 5.00%, Soybean oil 5.00%, Minerals 5.00%, Vitamins 1.00%, Colin 0.17%, and Lard 0%) while the rats in high-fat fed groups received high-fat chow 4.89 Kcal/gm (Casein 33.11%, Cystine 0.30%, Starch 15.21%, Dextrose 15.21%, Cellulose 5.00%, Soybean oil 5.00%, Minerals 5.00%, Vitamins 1.00%, Colin 0.17%, and Lard 20%) [27]. The diets were obtained from Faculty of Agriculture -Zagazig University. All the experimental procedures were conducted in accordance with the guiding principles for the care and use of research animals and were approved by the Institutional Research Board of Faculty of Medicine Zagazig University.
2.1. Determination of the Estrous Phases for Female Groups
- After 70th day of age, vaginal smears of all females were taken daily at 1PM and analyzed under the microscope and the mean frequency of diestrus, metestrus, proestrus and estrus [28, 29] was compared between the groups. The estrous cycle was analyzed for approximately three weeks (about 5 consecutive cycles) to assess estrous cycle before the start of obestatin administration. Then data were plotted in records of each labeled rat.The mean duration of the estrous cycle was 4-5 days in normal fed group and characterized as: proestrus, estrus, metestrus and diestrus, which was determined according to the cell types observed in the vaginal smear [28, 29], while in HFF group the mean duration was 9-11 days.Vaginal secretion was collected with a plastic pipette filled with 10 mL of normal saline (NaCl 0.9%) by inserting the tip into the rat vagina, but not deeply. One drop was collected with a clean tip from each rat and vaginal fluid was placed on glass slides. Unstained material was observed under a light microscope, without the use of the condenser lens, with 10 and 40 x objective lenses. Three types of cells could be recognized: round and nucleated ones are epithelial cells; irregular ones without nucleus are the cornified cells; and the little round ones are the leukocytes. The proportion among them was used for the determination of the estrous cycle phases according to [30, 31, 29] as follow:The proestrus phase: the vaginal smear consists of a predominance of nucleated epithelial cells with smooth margins.The estrus phase: the vaginal smear shows large anucleated cornified (keratinized) cells with irregular margins.The metestrus phase: the vaginal smear shows many cornified cells plus infiltration of leukocytes.The diestrus phase: the vaginal smear shows absence of the cornified cells and presence of small leukocytes.-To assess effect of obestatin injection on estrous cycle, vaginal smears were taken again daily and examined from the first day of obestatin injection until the last day before decapitation of female rats in all groups and the phases were assessed as mentioned before.
2.2. Anthropometric Measures
- Measuring body weight: by using a digital scale, the animal was weighed day before the experiment, twice a week and at the last day. The results were written in a record for each labeled rat.Measuring rat length: nose to anus length was measured at the start and the end of the experiment. The animal is allowed to move while an assistant was holding it from the tail to lengthen the body to ensure the real nose to anus length of the animal and avoid false measures. Metal ruler graduated in centimeters was used by holding zero end at the anus and record the reading that reached by the nose, then all measures were plotted for each labeled rat in its record [32].Measuring abdominal circumference (AC) and thoracic circumference (TC): by holding the measuring tape around the abdomen just in front of the hind limbs for AC recording and around the chest just behind the forelimbs for TC recording. Then data were plotted in records of each labeled rat [32].Calculating BMI index and AC/TC ratio: by using the previous data we calculated BMI which equals body weight (gm) / length2 (cm2), this index can be used as an indicator of obesity where the cutoff value of obesity BMI is more than 0.68 gm/cm2 [32] and divided AC by TC to calculate AC/TC ratio which is a measure of development of abdominal or visceral obesity [32].Blood collection: Overnight fasting animals were sacrificed after the end of experimental period under light ether anesthesia, and blood samples (8 ml/rat) were obtained by decapitation of all rats 5 days after stoppage of obestatin administration [26], and were collected in clean plastic centrifuge tubes and allowed to clot. Serum was separated by centrifugation of blood at 3000 rpm for 15 minutes. The supernatant serum was pipetted off using fine tipped automatic pipettes and stored frozen at -20ºC until assayed. Biochemical Analysis: 1) Serum glucose level: According to Tietz, [33] using glucose enzymatic (GOD-PAP)-liquizyme Kits (Biotechnology, Egypt), measured by using spectrophotometer (spectronic 3000 Array, Germany) at 546 nm. 2) Serum insulin level: according to Starr et al. [34] using rat insulin enzyme-linked immunosorbent assay kit (Product Number: RAB0904, Sigma-Aldrich Chemie GmbH, U.S.A).Calculation of homeostasis model assessment of insulin resistance (HOMA-IR): the following equation was used; [HOMA-IR = insulin (µU/mL) x glucose (mmol/L) / 22.5] [35]. 3) Determination of total serum cholesterol level: according to the method described by Flegg, [36] and Allain et al. [37] using rat cholesterol enzyme-linked immunosorbent assay kit (Catalog Number: 2011-11-0198, shanghai sunred biological technology, china). 4) Determination of serum triglycerides level: according to the method described by Nagele et al. [38] and Naito, [39] using rat triglycerides enzyme-linked immunosorbent assay kit: (Catalog Number: 2011-11-0250, shanghai sunred biological technology, china). 5) Determination of serum high density lipoprotein cholesterol level (HDL): according to the method described by Warnick et al. [40] using rat HDL-cholesterol enzyme-linked immunosorbent assay kit (Catalog Number: 2011-11-0255, shanghai sunred biological technology, china). 6) Determination of low density lipoprotein cholesterol (LDL) level: according to Friedewald et al. [41], LDL was calculated as follows: LDL=TC-HDL-TG\5. 7) Estimation of serum FSH level: according to the method described by Rebar et al. [42] using rat follicle-Stimulating Hormone (FSH) enzyme-linked immunosorbent assay kit: (Catalog Number: 2011-11-0183, shanghai sunred biological technology, china). 8) Estimation of serum LH level: according to the method described by Tietz, [33] using rat luteinizing hormone (LH) enzyme-linked immunosorbent assay kit: (Catalog Number: 2011-11-0180, shanghai sunred biological technology, china). 9) Determination of serum estradiol Level: according to the method described by Tietz, [33] using rat estradiol (E2) enzyme-linked immunosorbent assay kit: (Catalog Number: 2011-11-0175, shanghai sunred biological technology, china). 10) Determination of serum progesterone Level: according to the method described by Tietz, [33) using rat progesterone enzyme-linked immunosorbent assay Kit: (Catalog Number: Catalog Number: 2011-11-0742, shanghai sunred biological technology, china). 11) Determination of serum testosterone Level: according to the method described by Tietz, [33] using rat testosterone enzyme-linked immune-sorbent assay kit: (Catalog Number: 2011-11-5126, shanghai sun red biological technology, china).
2.3. Post Mortem Examination
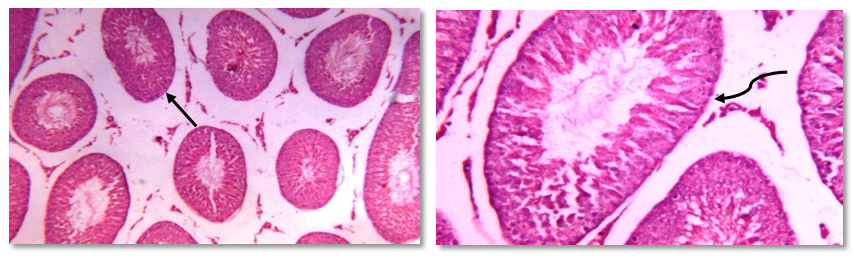
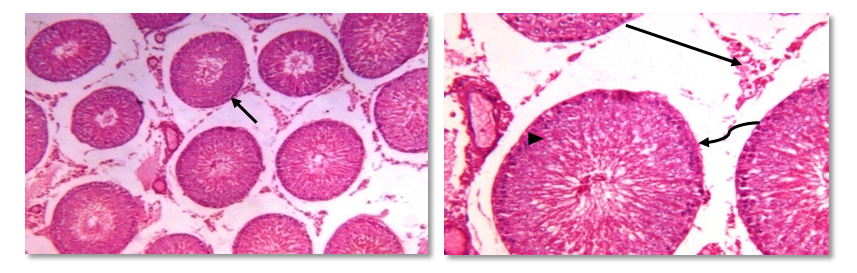
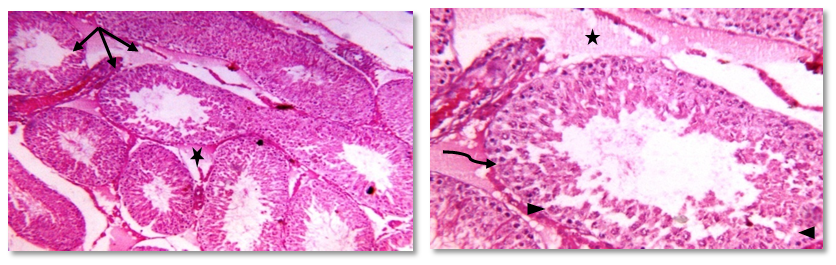
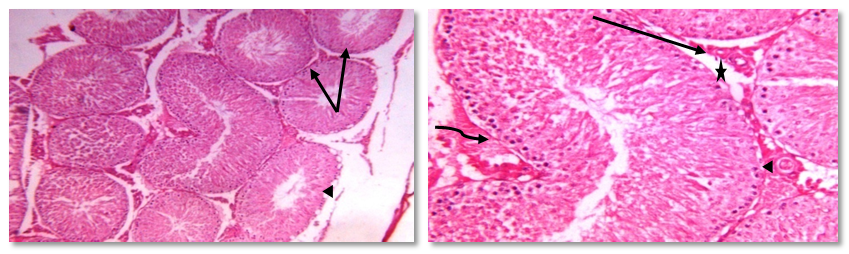
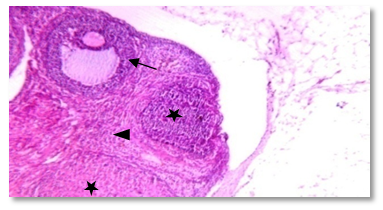
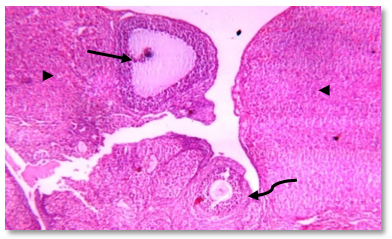
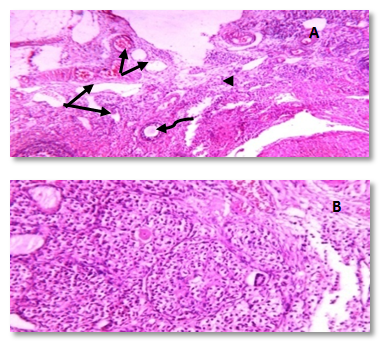
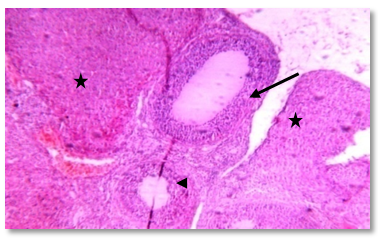
- 1) Extraction of fat and gonadal tissue: all animals were examined postmortem to determine the weights of mesenteric, epididymal, and periovarian fats in addition to right ovaries or testes, according to the sex. The abdominal wall was opened then the right gonads (according to the sex) were extracted by using one hand and dissection of the fat tissues by the other hand carefully. In addition to dissection of mesenteric fat [43]. 2) Spermatic parameters analysis: the right epididymis of each rat was dissected, removed and minced in 2 ml of Hank’s buffer salt solution (HBSS) at 37°C [44]. After 5 min incubation at 37°C, the cauda epididymis sperm was analyzed using the standard hemocytometric method. The epididymal fluid was drawn up to the 0.5 mark of WBC pipette (White Blood Cell pipette) and the semen diluting fluid (sodium bicarbonate 5 g, formalin 1 ml, distilled water 99.0 ml) was drawn up to '11' mark, and subsequently mixed well. One drop was added to the haemocytometer chamber and allowed the sperms to settle by keeping haemocytometer in humid place (wet chamber) for 1 h. After incubation the number of spermatozoa in the appropriate squares of the haemocytometer was counted under the light microscope at 400X. The sperm concentration refers to the number of spermatozoa / ml fluid, and calculated using the following formula. Sperm count =No. of spermatozoa counted x dilution factor x volume factor/ No. of areas counted [45]. The percentage of sperm motility was calculated using the number of live sperm cells over the total number of sperm cells, both motile and non-motile. The sperm cells that were not moving at all were considered to be non-motile, while the rest, which displayed some movement were considered to be motile [46]. 3) Gonadal histopathological examination: On the stipulated day after the collection of blood for hormonal assay, laparotomy was performed, and right testis and ovaries from all groups were removed and weighed followed by histopathological examination as follow: 1-Tunica vaginalis was carefully removed and the testis were dissected out and cleaned with cold physiological saline to remove blood and the adhering tissues. The samples were then fixed in 10% formaldehyde in fresh alcoholic bouin's fluid for 8 hours, and then processed and embedded in paraffain wax, sectioned at 5 µm thickness, and stained in hematoxylin-eoisin. The sections were examined or observed under a light microscope and the general histological appearance was assessed. The testis histology was performed according to the method used by [47, 48].2-The ovaries were dissected and fixed in 4% buffered paraformaldehyde at 4°C overnight and washed in a phosphate buffer saline solution. For light microscopy, fixed tissues were dehydrated in an ascending series of ethanol, cleared in xylene and embedded in paraffin. 5 μm thick sections were prepared. and stained with hematoxylin and eoisin (H&E) and histologically analyzed under a light microscope [49].
2.4. Statistical Analysis
- The data obtained in the present study were expressed as mean SD for quantitative variables and statistically analyzed by using SPSS program (version 18 for windows) (SPSS Inc. Chicago, IL, USA). One way Analysis of Variance (ANOVA) was used to compare the results of all examined groups followed by LSD test to compare statistical differences between groups. P value <0.05 was considered statistically significant.
3. Results
- This study revealed that high fat diet in both male and female rats significantly (p<0.001) increased body weight, body mass index, AC/TC ratio, mesenteric fat, serum glucose& insulin levels, and HOMA-IR. Moreover, it was found that exogenous administration of obestatin resulted in a significant (p<0.001) decrease of all the above mentioned parameters except BMI (p<0.01), in the HFF group (group IIb). In the normal fed group (group Ib), obestatin produced a significant decrease in body weight (p<0.001), body mass index (p<0.01 in males and P<0.001 in females), AC/TC ratio (p<0.01, p<0.05 in male and female rats, respectively) and serum glucose levels (p<0.05), while it was associated with a significant increase in serum insulin level (p<0.01). Also, it was recorded from the results of this study that HFD resulted in dyslipidemic changes as illustrated by a significant increased (p<0.001) in serum levels of triglycerides, total cholesterol, LDL cholesterol. On the other hand, serum HDL cholesterol was significantly decreased (p<0.001) as compared to (group Ia). Moreover, it was found that exogenous administration of obestatin in both normal fed (group Ib) and HFF (group IIb) significantly decreased serum levels of total cholesterol, triglycerides , and LDL cholesterol. On the other hand, serum HDL cholesterol levels were significantly increased (p<0.001, p<0.01 in male and female rats, respectively) as compared to saline treated groups (group Ia and group IIa, respectively) (tables 1 & 2).
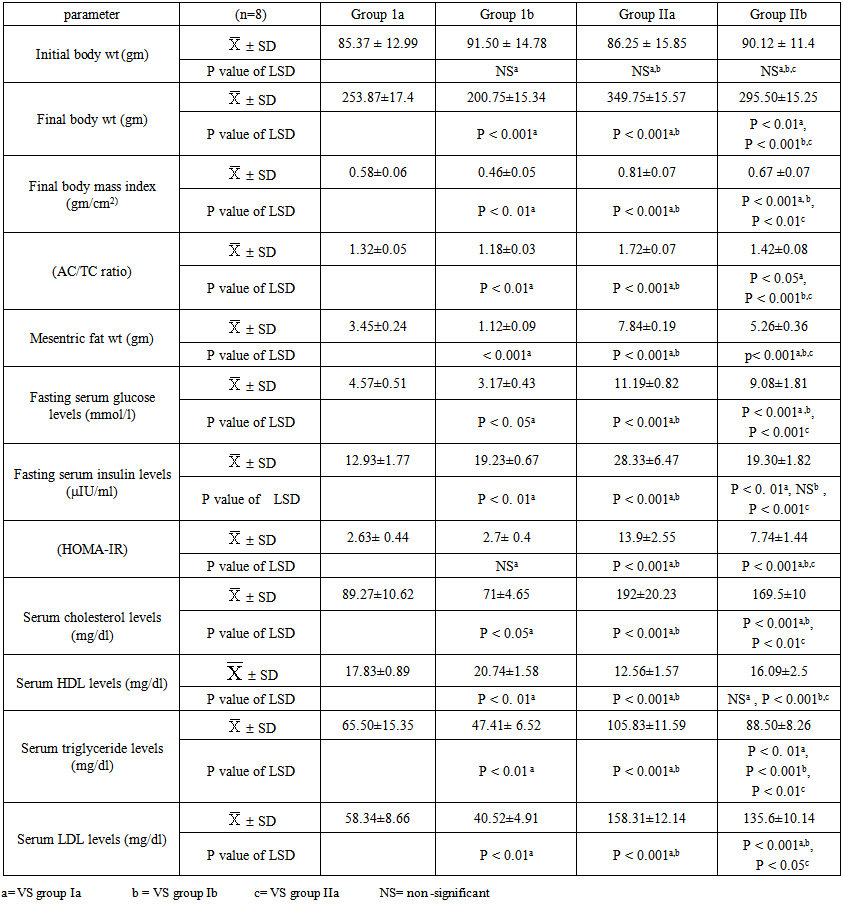 | Table 1. The anthropometric and metabolic parameters in males of all studied groups |
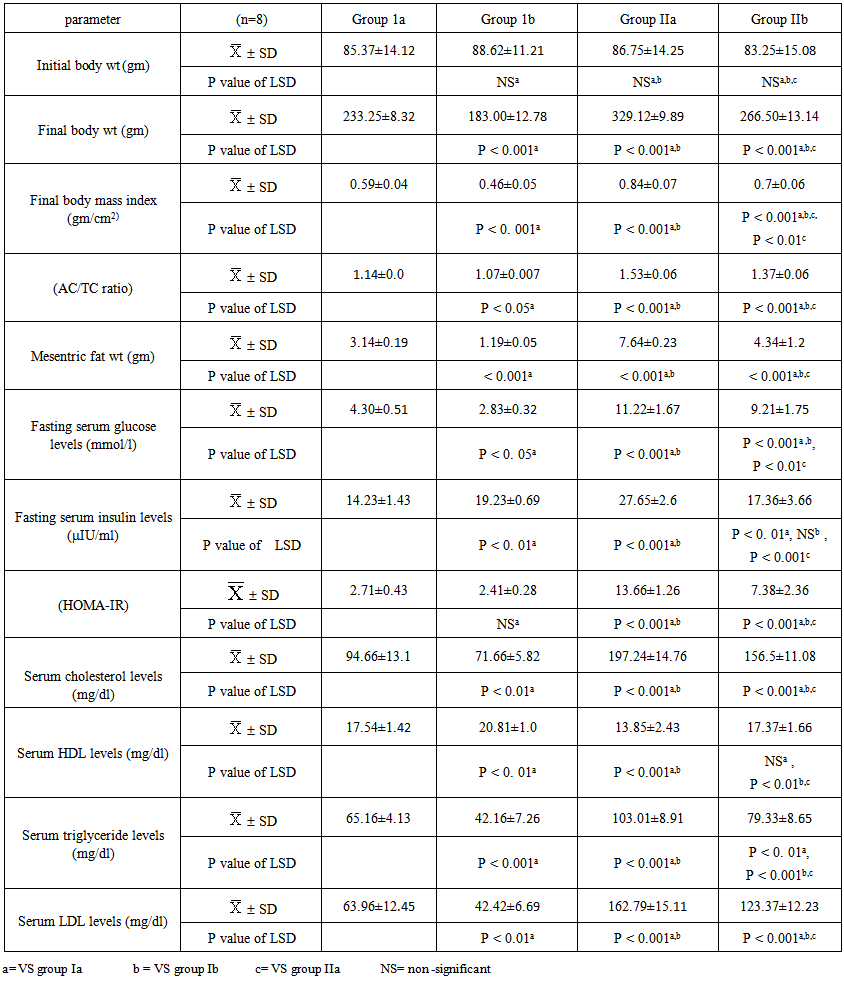 | Table 2. The anthropometric and metabolic parameters in females of all studied groups |
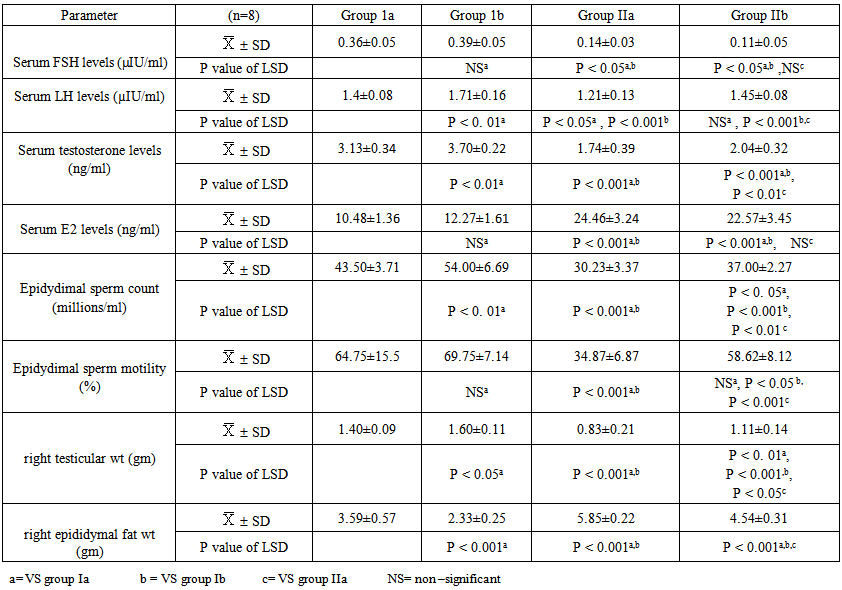 | Table 3. Serum FSH, LH, Testosterone, E2 levels, epididymal sperm count & motility and right testicular & epididymal fat weights in males of all studied groups |
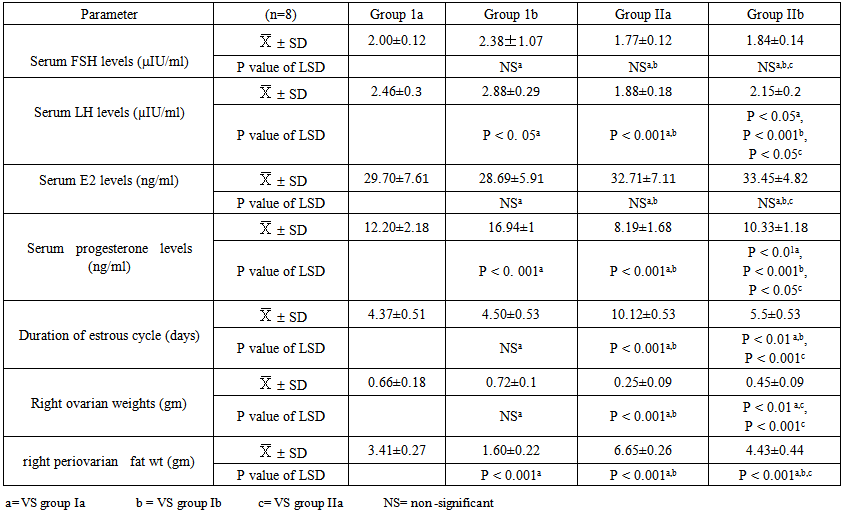 | Table 4. Serum FSH, LH, E2, progesterone levels, duration of estrous cycle, right ovarian & periovarian fat weights in females of all studied groups |
4. Discussion
- Human studies have shown a direct relationship between obesity and infertility in association with metabolic disturbances and hormonal dysregulation [50, 51]. Recently, obestatin was reported as a novel adipocytokine [52], and its levels were decreased in obese subjects [53, 54].Therefore the objective of the current work was to explore the probable effects of this peptide in modulating the adverse effects of obesity on gonadal functions with a trial to clarify some of the possible underlying mechanisms.The results of this study revealed that, administration of HFD since weaning was able to induce obesity and negatively alter gonadal function in adult rats.In HFF male group a significant reduction in serum levels of LH, FSH, testosterone, and elevation of serum E2 levels were reported. Furthermore, the present findings showed that high fat diet resulted in marked reduction in epididymal sperm count and motility together with a significant decrease in testicular weight, and a significant increase in epididymal fat in the same group (IIa) when compared to the normal fed group (Ia). These effects which explain the association between reduction in fertility and obesity are supported by the findings of other investigations in both animals and humans [55, 3, 56, 57, 7, 58].Indeed, increased conversion of testosterone to oestradiol in the adipose tissue in case of obesity might contribute to decreased plasma testosterone concentrations and lead to secondary hypogonadism, as estrogen receptors are present in hypothalamic nuclei and in pituitary gonadotropes, it is thought that estrogen acts on the hypothalamus to affect gonadotrophin realsing hormone (GnRH) pulses and leads to suppression of the hypothalamic-pituitary axis resulting in subclinical hypogonadotropic hypogonadism [59]. Other authors suggest that estradiol could have a direct effect on testicular environment altering the spermatogenesis [60-62].In addition, circulating hyperleptinemia associated with obesity is known to have a deleterious effect on androgen production via a central mechanism [63]. Furthermore, leptin might act as a paracrine agent in testis to regulate testosterone production by acting directly on testicular Leydig cells where this hormone interferes with testicular steroidogenesis via decreasing gene expression of several factors involved in the steroidogenic pathway [64] and this explain the link between decreased testosterone secretion and hyperleptinemia in obese men [65].Insulin is another factor that plays a regulatory role in reproductive function. In experimental animals, both acute insulin deprivation and insulin-driven reductions in brain stem glucose availability dampen LH pulsatility and impair acute gonadotrope secretory responsiveness to GnRH [66, 67]. Moreover, as a consequence of reduced insulin action, Leydig cell function may be compromised and testicular steroidogenesis impaired, leading to decreased circulating testosterone concentrations [68].In fact, it has been shown that saturated fatty acid treatment decreases LH-stimulated adenylate cyclase activity and testosterone levels in rat testis [69], and induces apoptosis of Leydig cells [70]. Other additional factors that may potentially decrease LH secretion in obese animals are glucocorticoids, which have been found elevated in high-fat diet fed rats [71-73].As the apparently paradoxical coexistence of low serum testosterone and reduced levels of circulating LH induced by the high-fat diet can be explained by several mechanisms including reduced GnRH output from the hypothalamus and impaired action of this releasing peptide at the pituitary level, both can be resulting from insulin resistance [74, 67, 75, 69, 70, 72].Moreover, the decreased level of FSH may be included also, since this hormone is essential to stimulate Sertoli cells to synthesize and secrete androgen-binding protein, which is important to bind testosterone that is required for terminal differentiation of spermatids [76]. In addition, decreased FSH level would also decrease the production of the stem cell factor (SCF). This factor is a Sertoli cell product that has been involved in Leydig cell development and survival and is acting as a survival factor for the different cell types in the seminiferous epithelium such as spermatogonia in adult rats [77].Codoner-Franch et al. [78] noted that excess adiposity and the resultant dyslipidemia are associated with systemic proinflammatory states and increased oxidative stress (OS) and reactive oxygen species (ROS) in obese rats [79]. It is well established that hyperglycemia also elicits an increase in ROS production [80].The major targets of ROS are membrane lipids and this process is called lipid peroxidation. It is also known that, the testicular tissues and spermatozoa are very sensitive to ROS attack and lipid peroxidation. Susceptibility of testicular tissues to oxidation was attributed to the highly rich polyunsaturated fatty acid content of sperm membranes [81].Possible physiopathogenic explanations for the OS in the epididymis could be due to excess fat deposit in the heads of the epididymidis, ‘‘epididymal lipomatosis’’, with reduction in the antioxidant system, which in turn reduces capacity to eliminate ROS [82]. Oxidative stress stimulating macrophages and other inflammatory cells to secrete cytokines as IL 1 β & TNF, and nitric oxide (NO) which affect both steroidogenesis and spermatogenesis [83, 78, 84].Vigueras-Villasenor et al. [27] observed the phenomenon of increased apoptosis mediated by OS in the heads of the epididymidis in obese rats. This could have an impact on the epididymal function [85] and resulted in a low sperm count, viability and motility [55, 4, 5].In the present study, we could demonstrate a clear relationship between obestatin administration and male gonadal functions as exogenous obestatin administration resulted in a significant increase in serum levels of LH & testosterone and epididymal sperm count in both normal fed and HFF groups and sperm motility in HFF group together with a significant increase in testicular weight, and a significant decrease in epididymal fat in both groups (Ib,IIb) when compared to their saline treated groups (Ia, IIa).The observations provided evidence for an involvement of obestatin in enhancing the testosterone production from leydig cells. It is either that this increase in the testosterone secretion is the direct result of the binding of obestatin to GPR39 which is present in testes [86), another explanation may be involved is that obestatin could have enhanced the responsiveness of the leydig cells towards pituitary LH [26].Furthermore, Allwsh and Mohammad, [87] showed that there was a significant decrease in serum Malondialdhyde (MDA) level in obestatin injected groups compared to control groups. The reason might be due to the ability of obestatin to restore oxidative balance and decrease oxidative stress. So, it can be concluded that, obestatin can exert protective effect against oxidative stress either by itself [88], or the cause might be attributed to the antioxidant properties of adiponectin [89], which correlated positively with obestatin [87].Our findings are proved by the results of the histopathology of testicular sections in HFF male group (IIa) which showed that: seminiferous tubules were variable in size and shape with atrophy in their walls and reduction of sperm Lineage cells. Moreover, walls of tubules were vacuolated with destruction of sertoli cells and increased thickness of the basement membrane, which were embedded in oedematous interstitial tissue. While exogenous obestatin administration was associated with a marked improvement in testicular histoarchitecture in HFF group and significant increase in the spermatogenic activity in both normal fed and HFD fed groups.This is in accordance with the findings of Jahan et al. [26] who reported the stimulatory effect of this peptide on testosterone secretion and that elevated testosterone level might have directly enhanced the spermatogenesis as observed in majority of the seminiferous tubules in the treated animals when compared with the control animals in their study.We also reported the effect of high fat diet on female rat gonadal functions. The HFD in group (IIa) resulted in a significant reduction of serum levels of LH & progesterone together with a significant increase in estrous cycle duration. Furthermore, weight of the ovaries was significantly decreased and periovarian fat weight was significantly increased in the same group when compared with that of normal diet fed group (Ia).This is in accordance with the findings of Sagae et al. [13] who reported that obesity can negatively alter reproductive function in adult female rats. Also, Jerome et al. (90) and Honnma et al. [91] reported that estrous cyclicity was abnormal with prolongation of diestrus in fatty rats.Hyperinsulinemia could be contributing to the reduced ovulation in obese rats. An excess of insulin may operate at the pituitary level to dampen the LH pulse amplitude. In addition, this excess contributes to reduced sex hormones binding globulin (SHBG) concentrations proportionally with its blood levels [92]; as such, excess insulin may further increase the delivery of free androgens and estrogens to the target. The excess of local ovarian androgens promotes follicular atresia in rats and inhibits follicular growth and development [74].Additionally, a fat-derived factor, such as leptin could also be contributing to favor anovulation, and menstrual cycle irregularity. Obese female rats present high levels of leptin and studies have evidence the important role of leptin on reproductive alterations in obesity [93, 94]. Furthermore, in vitro and in vivo studies show that high leptin levels in ovary may interfere in the development of dominant follicles and oocytes maturation [95, 96].Lin et al. [94] noticed that leptin influence gonadal functions through modulating steroidogenesis in granulosa cells. In particular, it has been found that leptin antagonizes insulin action in human granulose cells and thereby inhibits their gonadotropin-stimulated progesterone production [97], as leptin acts through the MAPK pathway to down regulate cAMP-induced StAR (steroidogenic acute regulatory protein) expression and progesterone production from granulosa cells [94].The results of this work also showed that, obestatin administration significantly increased serum levels of LH and progesterone in both normal and HFF groups. Furthermore, exogenous obestatin administration resulted in a significant decrease in estrous cycle duration, increase in ovarian weight and decrease in periovarian fat in HFF group (IIb) when compared to its control group (IIa). But no significant changes were observed in normal fed group (Ib) as a result of obestatin administration except the significant decrease in periovarian fat when compared to its control group (Ia).Our findings are supported by those who reported that, the obestatin-induced secretion of progesterone represents the evidence for an involvement of obestatin in the control of ovarian hormone secretion. As only progesterone, the dominant steroid hormone of the corpus luteum, was affected it can be proposed that obestatin is a stimulator of ovarian follicular cell luteinization. It seems not to affect the androgen and estrogen which would be involved in ovarian follicular development and atresia [98-100]. Whatever, Meszarosova et al. [25] observed that obestatin stimulates the proliferation of porcine granulose cells through promotion of the M-phase of the cell cycle.Intracellular mechanisms of obestatin action on the ovary include c-AMP/protein kinase A-dependent intracellular signalling pathways in mediating its action via GPR39 [14]. Another protein involved is MAPK (mitogen activated protein kinase), which is not only a marker of proliferation but also an intracellular mediator of effect of some growth factors. These observations provide evidence for an involvement of obestatin in the direct control of ovarian cell functions (proliferation, apoptosis and secretory activity) [101].In the present study, the histopathology of ovarian sections in HFF (IIa) group showed many cystic follicles, which were intensively observed with an ovarian stroma of vacuolated appearance with lipid droplets. Together with reduction in diameter of primary follicle and many atretic follicles. This is in accordance with the findings of Honnma et al. [91] and Wang et al. [49] who found that ovaries from fatty rats showed attenuated follicle growth and multiple cysts within the interstitial tissue. They also reported that, follicular atresia is accelerated in fatty rats, suggesting that obesity may promote follicle apoptosis.While, the histopathology of ovarian sections in HFF (IIb) group showed that exogenous obestatin administration was associated with a marked reduction in number of cystic and atretic follicles which were embedded in normal appearance ovarian stroma, which in turn proved the beneficial role of obestatin in the same group.Regarding the anthropometric and the metabolic changes, this study revealed that high fat diet in both male and female rats significantly increased body weight, body mass index, AC/TC ratio, mesenteric fat, serum glucose& insulin levels, and HOMA-IR. Moreover, it was found that exogenous administration of obestatin resulted in a significant decrease of all the above mentioned parameters in the HFF group (IIb). In the normal fed group, obestatin administration was associated with a significant decrease in body weight, body mass index, AC/TC ratio, mesenteric fat, and serum glucose levels, while there was a significant increase in serum insulin level.This weight reducing effect of obestatin in both normal fed and HFF group is in accordance with the findings of Lagaud et al. [102], Samson et al. [103], Hassouna et al. [21] and mony et al. [104] who reported that obestatin suppressed food intake, body weight gain, gastric emptying and intestinal motility through an interaction with the orphan receptor GPR39 [20].Furthermore, Guo et al. [53] reported lower level of preprandial obestatin in obese patients compared with normal weight individuals, which might be related to the disturbed satiety perception in obesity and anorexigenic effect of obestatin. However, the role of obestatin as anorexigenic hormone is not clearly understood [21]. Obestatin may exert its effect through central action opposing the foregut-induced orexigenic effect of ghrelin on food intake [105]. As obestatin inhibits jejunal contractile activity and suppresses gastric emptying, it cannot be excluded that its anorexigenic effect relies on the peripheral sites of action [106].In contrast, other investigators showed no significant difference between high fat diet (HFD) fed rats untreated and treated with obestatin in body wt gain [107].As regard serum glucose levels, our findings are in line with those of Allwsh and Mohammad, [87] who reported that intraperitoneal (i.p) injection of obestatin in both normal and diabetic rats caused a significant decrease in serum glucose level compared to the control groups, and also with other researchers who reported an inverse relationship between circulating levels of obestatin and plasma glucose levels in obese rats [23, 108, 24].The glucose lowering effect in our study could be attributed to the findings of Granata et al. [26] who found that obestatin stimulated glucose uptake per se and enhanced the effect of insulin in both 3T3-L1 and human subcutaneous (hSC) adipocytes. In this regard, obestatin promoted glucose transporter-4 (GLUT4) translocation to the plasma membrane in 3T3-L1 adipocytes. This effect was at least as strong as that of insulin, suggesting that obestatin may influence GLUT4 translocation and glucose uptake independently of insulin. Also, it induced Akt (Serine, threonine protein kinase) phosphorylation and activated downstream targets of insulin in both pancreatic β cells and adipocytes [109].Regarding serum insulin levels in normal fed (group Ib), our findings are in agreement with the findings of Granata et al. [106] who demonstrated that in human islets, obestatin promoted B cell survival and blocked cytokine-induced apoptosis through cAMP increase and involvement of adenyl cyclase/cAMP/PKA (Protein kinase A) signaling. They also reported that, obestatin induces phosphorylation of a group of mediators like: PI 3-kinase/Akt, ERK (Extracellular signal-regulated kinase) 1/2, and also cAMP response element–binding protein, which in turn stimulates insulin secretion and gene expression.On the other hand, Mony et al. [104] reported an inhibitory effect of exogenous obestatin on serum insulin in rats.This controversy may be due to differences in chosen dose of obestatin, nutritional or the metabolic state of the animal.Concerning insulin resistance in obestatin treated HFF groups, our results revealed that obestatin markedly improved insulin sensitivity as indicated by the significant decrease in HOMA-IR, which in turn was associated with a significant decrease in serum insulin levels.Findings of the current work are in line with those of Granata et al. [51] who observed obestatin insulin- sensitizing effects as they found that, obestatin increased Akt and adenosine monophosphate-activated protein kinase (AMPK) phosphorylation in white adipose tissue (WAT), muscle and liver of HFD-fed mice. In fact, Akt and AMPK play a key role in peripheral insulin sensitivity, and their activity is reduced in humans and in animal models of insulin resistance [110-112].Furthermore, Granata et al. [51] observed that obestatin completely blocked HFD-induced tumor necrosis factor-α (TNF-α) increase in WAT, liver, and muscle and similarly reduced interlukien-1β (IL-1β) in WAT and liver. Therefore, they concluded that alleviated insulin resistance observed in obestatin-treated mice fed a HFD may also result from reduced inflammation in peripheral tissues.Moreover, Kadowaki et al. [113] observed that obestatin increased adiponectin and reduced leptin secretion from hSC adipocytes in HFD-fed mice. Adiponectin is known to improve insulin sensitivity by increasing energy expenditure and fatty acid oxidation [114]. Conversely, leptin impairs insulin metabolic actions in adipocytes, i.e., stimulation of glucose transport and lipogenesis [115].The next metabolic effect in this work was the effect of high fat diet in both male and female rats on lipid profile. It was recorded from the results of this study that HFD in group (IIa) resulted in dyslipidemic changes as illustrated by increasing serum levels of total cholesterol triglycerides, LDL cholesterol. On the other hand, serum HDL cholesterol was significantly decreased as compared to normal fed rats (Ia). Moreover, it was found that exogenous administration of obestatin in both normal (Ib) and HFF (IIb) rats significantly decreased serum levels of total cholesterol, triglycerides and LDL cholesterol. On the other hand, serum HDL cholesterol levels were significantly increased as compared to controls (group Ia & IIa, respectively). This finding is in line with that of Woo et al. [116], and Kamal and Mohamed, [117].These generally increased levels of serum lipids may be mainly attributed to increase in the mobilization of free fatty acids from fat depots. Then, excess fatty acids in serum are converted into triglycerides, phospholipids and cholesterol in liver [118-121].Concerning the effect of obestatin administration on serum lipid profile our findings are in agreement with those obtained by other investigators who revealed that there were a significant decrease in total serum cholesterol, triglycerides, LDL-C and a significant increase in serum HDL-C levels in obestatin injected groups compared to control groups [87]. Also, Nagaraj et al. [122, 123] and Angew et al. [124] showed a negative significant correlation between obestatin and triglycerides level.This effect of obestatin on lipid profile might be due to the role of this peptide in inhibiting food intake, gastric emptying and gastrointestinal motility [14]. Also, this reduction might be due to that obestatin increase the phosphorylation of AMPK [125] and then inhibit 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMG-CoA reductase) which contributes in biosynthesis of cholesterol [124]. Also, the phosphorylation of AMPK will inhibit acetyl CoA carboxylase which contributes in biosynthesis of fatty acid and triglycerides [110].The cause of increased serum HDL cholesterol might be due to that obestatin stimulates insulin secretion and sensitivity as proved in our study, so the activity of lipoprotein lipase will be increased and leads to increase HDL-C [105]. Furthermore, obestatin induced phosphorylation of AMPK and this will reduce inflammatory markers such as TNF-α and IL-6 [88] and lead to induction of adiponectin which correlates positively with HDL-C [126, 87].
5. Conclusions
- It could be suggested that obestatin has a potential positive role against obesity-induced gonadal dysfunction, which may be due to its role in maintenance of glucose & insulin homeostasis, and /or maintenance of gonadal hormonal function via indirect and/or direct effect on the gonad. Further studies are required to investigate the potential therapeutic effect of obestatin in case of obesity associated reproductive dysfunction and to explore the possible involved mechanism/s.Finally, our data suggest obestatin as a metabolic hormone could provide new explanations for the interrelationship between metabolism, nutrition and reproduction, as well as new approaches for controlling these processes in the treatment of reproductive and metabolic disorders.
ACKNOWLEDGMENTS
- To Prof./Kamal EL-Kashish, pathology department - Medicine, Zagazig University for performing the histological study and to Prof. / Somiaa Hassan Biochemistry department- Medicine, Zagazig University for performing the laboratory tests.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML