-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2014; 3(3): 37-42
doi:10.5923/j.medicine.20140303.01
Effects of Aqueous Extract of Moringa oleifera Seeds on Alloxan Induced Hyperglycemia
Meraiyebu Ajibola, Ogunwole Eunice, Izuchukwu Nnnedinma Stephanie
Department of Physiology, College of Health Sciences, Bingham University New Karu, Nasarawa, P.M.B 005. Nigeria
Correspondence to: Ogunwole Eunice, Department of Physiology, College of Health Sciences, Bingham University New Karu, Nasarawa, P.M.B 005. Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
This study examines the effect of aqueous extract of MoringaOleifera seeds on alloxan induced mild and severe hyperglycemia relative to its route of administration. The animals were divided into six groups (n = 6). Group I animals received only rat chow and water (served as control group). Group II animals received only alloxan and were not treated (served as Hyperglycemic group), Group III received alloxan and after three hours were treated with 400mg/kg body weight of aqueous extract of moringaoleifera seed orally (served as mild hyperglycemic group A), Group IV received alloxan and after three hours were treated with 400mg/kg body weight of aqueous extract of moringaoleifera seed intraperitoneally (served as mild hyperglycemic group B). Group V received alloxan and after two days, began receiving treatment with 400mg/kg body weight aqueous extract of moringaoleifera seed orally for two weeks (served as severe hyperglycemic group A). Group VI received alloxan and after two days, began receiving treatment with 400mg/kg body weight aqueous extract of moringaoleifera seed intraperitoneally for two weeks (served as severe hyperglycemic group B). The result of the study showed a significant decrease in the blood glucose level after six hours and also after fourteen days of both oral and intraperitoneal treatment of the mild hyperglycemia with moringaoleiferaseed extract. Also there was a 48.6% and 42.8% decrease in the blood glucose level of the mildly hyperglycemic rats on treatment with both oral and intraperitoneal moringaoleifera seed extracts and a 69.7% and 89.6% decrease in the blood glucose level of the severely hyperglycemic rats on treatment with both oral and intraperitoneal moringaoleifera seed extracts respectively. The study shows that Moringa oleifera seed extract exhibited a hypoglycemic effect on both the mild and severe alloxan induced hyperglycemic rats.
Keywords: Alloxan, Hyperglycemia, Moringa oleiferaseed, Rats
Cite this paper: Meraiyebu Ajibola, Ogunwole Eunice, Izuchukwu Nnnedinma Stephanie, Effects of Aqueous Extract of Moringa oleifera Seeds on Alloxan Induced Hyperglycemia, Basic Sciences of Medicine , Vol. 3 No. 3, 2014, pp. 37-42. doi: 10.5923/j.medicine.20140303.01.
Article Outline
1. Introduction
- Moringa Oleifera (drumstick or horseradish in English) is a member of Moringaceae family. It is an edible plant and it is grown extensively in many Southeast Asian countries particularly in Thailand, India, Philippines and Pakistan [1]. It has long been known as a food plant and as an ingredient of Indian traditional medicine [2, 3]. Phytochemical analyses have shown that its leaves are particularly rich in potassium, calcium, phosphorous, iron, vitamins A and D, essential amino acids, as well as such known antioxidants such as β-carotene, vitamin C, and flavonoids [4, 5]. Aside the nutritional benefits of Moringa oleifera it has been reported to have medicinal values. Moringa oleifera has anti-inflammatory [6] and thyroid status regulator [7] efficacies and researchers reported its hypoglycemic potential [8]. Hyperglycemia can be defined as excess sugar (glucose) in the blood. It is a common complication of critical illness, regardless of a history of diabetes mellitus. It has an estimated prevalence of approximately 40% in hospitalized patients [9]. Hyperglycemia resulting either due to defective production or action of insulin leads to a number of complications; cardiovascular, renal, neurological, ocular etc [10]. In general, the normal range of glucose for most people (fasting adults) is about 80 to 110 mg/dl or 4 to 6 mmol/l. (where 80 mg/dl is "optimal".) An individual with a consistent range above 126 mg/dl or 7 `mmol/l is said to have hyperglycemia, whereas a consistent range below 70 mg/dl or 4 mmol/l is considered hypoglycemic. In fasting adults, blood plasma glucose should not exceed 126 mg/dL. An individual is diagnosed as diabetic when his blood glucose level is chronically ≥126 mg/dL after an overnight fast and ≥200 mg/dL 2h after an oral glucose load of 75g Oral (Glucose Tolerance Test, OGTT [11]. Sustained higher levels of blood sugar cause damage to the blood vessels and to the organs they supply, leading to the complications of diabetes [12]. Blood glucose levels can also get too high if cells are unable to respond to insulin properly (insulin resistance). Diabetes mellitus is a disease that occurs when the body can't use glucose properly. Hyperglycemia is a symptom of diabetes; however, one can have hyperglycemia without having diabetes.Alloxan is a drug use to induce experimental diabetes due to the selective destruction of the insulin-producing pancreatic beta – islets [13]. Alloxan induces a multiphasic blood glucose response when injected into an experimental animal, which is accompanied by corresponding inverse changes in the plasma insulin concentration followed by sequential ultrastructural beta cell changes ultimately leading to necrotic cell death [14]. In one of the multiphasic phases of alloxan, it has shown that one hour after the administration of alloxan, there is a rise in blood glucose level with a decrease in insulin concentration at the same time. This is known as the first hyperglycaemic phase after first contact of the alloxan with the pancreatic beta cells. This hyperglycaemic phase last for about 2-4 hours. [15]. This study therefore aimed to determine the anti-hyperglycemic activity of Moringa oleifera seed extract on alloxan induced mild and severe hyperglycemia in rats.
2. Methodology
2.1. Collection of Plant Material and Extraction
- 25g of the seeds of the plant Moringa oleifera bought from Masakka market in Nasarawa state of Nigeria was shade dried, milled and ground into coarse powder using a laboratory mortar. The coarse powder seed was soaked with 100ml of distilled water for 20min and filtered with a sieve. The filtrate was then centrifuged and the supernatant decanted, collected in a storage bottle, and stored at temperature of -4℃.
2.2. Acute Oral Toxicity Study of M. oleifera Extract
- Acute toxicity study was carried out according to Organisation for Economic Co-operation and Development (OECD) guideline 423 [29]. None of the rats showed observable signs of toxicity upon single administration of M. oleifera seed extract (2000mg/kg body weight) on day one. Observations twice daily for 14 days also did not reveal any drug related observable changes. A dose of 400mg/kg body weight was used for the study which is one fifth of the dose used for the toxicity study. It has been reported to have anti-hyperglycemic properties by increasing blood glucose tolerance in the normal rat [30].
2.3. Animals
- 36 Healthy adult female albino Wistar rats weighing between 200g - 225g were procured from National Institute of Pharmacological and Research Development (NIPRED). The animals were housed under laboratory conditions (12h light and 12h dark cycle). They were fed with rat chow and water ad libitum and acclimatized for two weeks.
2.4. Induction and Treatment of Mild and Severe Hyperglycemia
- The baseline blood glucose levels were determined before the induction of hyperglycemia. The rats were fasted overnight prior to injection of alloxan dissolved in normal saline at a dose of 150mg/kg body weight given intraperitoneally [23]. After 3 hours, a period that targets the first hyperglycaemic phase after the first contact of the pancreatic cells with the toxin [24, 25], rats with blood glucose levels greater than 135mg/dl were considered as mildly hyperglycaemic. While after 3days, a period that targets the last and 4th phase of the blood glucose response [24, 25, and 26] rats with blood glucose levels greater than 135mg/dl were considered as severely hyperglycemic and were used for the investigation [16]. The treatment with 400mg/kg body weight aqueous extract of moringa oleifera seed extract [27, 28] was done for fourteen days in which blood glucose levels and body weight of the rats were taken on day 0, 7 and 14 of administration.
2.5. Experimental Design
- The rats were randomly divided into 6 groups consisting of 6 animals each:Group I Control animals received only rat chow and waterGroup II Hyperglycemic animals that received only alloxan and no treatment Group III (mild hyperglycemic group): Animals received alloxan and after three hours were treated with 400mg/kg body weight of aqueous extract of moringa oleifera seed orally.Group IV (mild hyperglycemic group): Animals received alloxan and after three hours were treated with 400mg/kg body weight of aqueous extract of moringa oleifera seed intraperitoneally.Group V (severe hyperglycemic group): Animal received alloxan and after two days, began receiving treatment with 400mg/kg body weight aqueous extract of moringa oleifera seed orally for two weeks.Group VI (severe hyperglycemic group): Animal received alloxan and after two days, began receiving treatment with 400mg/kg body weight aqueous extract of moringa oleifera seed intraperitoneally for two weeks.
2.6. Measurement of Blood Glucose Level
- Blood glucose levels were measured by the Medisense Precision PCx (Abbott Medisense Division, Bedford, MA) blood glucose testing system (glucose strip method). The tip of the tail was snipped with sharp scissors and gently squeezed for a drop of blood. The strip was inserted into the machine, and the drop of blood was placed on the strip. Within 20s, the instrument measured and displayed the blood glucose level. The blood glucose level of the rats were taken just before the administration of alloxan (for the group that received alloxan) to induce mild and severe hyperglycemia.For Group III and IV, their blood glucose level was checked three hours after inducing hyperglycemia, before the total damaging of the pancreatic beta cells by the alloxan and was immediately treated with aqueous extract of moringa oleifera seed orally and intraperitoneally respectively in other to see the immediate effect of the extract on hyperglycemia before the complete damaging of the beta cells of the pancreas and also to compare the effectiveness of both routes of administration in treatment. The blood glucose level of the treated groups was observed three hours after treatment.For Group V and IV, their blood glucose level were checked two days after the administering alloxan after which they began receiving treatment with aqueous extract of moringa oleifera seed orally and intraperitoneally respectively for two weeks. A weekly record of the blood glucose was observed after oral and intraperitoneal administration where the rats had already developed hyperglycemia.
2.7. Statistical Analysis
- The data were expressed as mean ± S.E.M. Data were analysed using Student t-test and ANOVA was used for more than two groups. Data were considered significant when p < 0.05.
3. Results
- Table 1 show a significant decrease in the blood glucose level after six hours of both oral and intraperitoneal treatment of the mild hyperglycemia (that was induced by alloxan) with moringa oleifera seed extract.
|
4. Discussion
- Induction of diabetes using alloxan has been described as a useful experimental model for studying the effects of hypoglycemic agents [13]. Alloxan and the products of its reduction, dialuric acid, establish a redox cycle with the formation of superoxide radicals. These radicals undergo dismutation to hydrogen peroxide with simultaneous massive increase in cytosolic calcium concentration, resulting in the distruction of pancreatic beta cells and severe hyperglycemia [13]. The untreated hyperglycemic rats had a significantly higher fasting blood glucose level than control rats that received only rat chow and water ad libitum. This confirms induction of hyperglycemia by alloxan. Table 1 showed that in the mildy hyperglycemic rats, only a little increase in the fasting blood glucose was noted.Alloxan induces a multiphasic blood glucose response when injected into to an experimental animal, which is accompanied by corresponding inverse changes in the plasma insulin concentration followed by sequential ultrastructural beta cell changes ultimately leading to necrotic cell death. The first phase that comes into view within the first minutes after alloxan injection is transient hypoglycemic phase that lasts maximally for 30 minutes. The 2nd phase appearing one hour after administration of alloxan leads to rise in blood glucose concentration. Moreover, the plasma insulin concentration has been noted to decrease at the same time. This is the first hyperglycemic phase after the first contact of the pancreatic beta cells with the toxin. This hyperglycemic phase lasts for 2-4 hours which is accompanied by decreased plasma insulin concentrations. These changes are a result of inhibition of insulin secretion from the pancreatic beta cells that is attributed to the induction due to their beta cell toxicity [23, 24, and 27]. From this study it is suggested that moringa oleifera seed extract was able to reverse the inhibition of insulin secretion from the pancreatic beta cells since only a few of the pancreatic beta cells may have been destroyed before treatment with the extract began three hours after induction of hyperglycemia with alloxan compared to the control group.The 3rd phase is again a hypoglycemic phase that is noted 4-8 hours after the alloxan injection, which lasts for several hours. The flooding of circulation with insulin occurs as a result of the alloxan-induced secretory granule and cell membrane rupture resulting in severe transitional hypoglycemia. In addition, other subcellular organelles are also ruptured that include cisternae of rough endoplasmic reticulum and the golgi complex. Moreover, the outer and inner membranes of the mitochondria loose structural integrity in this particular phase. These changes are irreversible and highly characteristic for a necrotic cell death of pancreatic islets [25].The last and the 4th phase of the blood glucose response is the final permanent diabetic hyperglycemic phase during which complete degranulation and loss of the integrity of the beta cells within 24-48 h after administration of the alloxan takes place [26]. The extract demonstrated antihyperglycemic effect by causing a significant decrease in the fasting blood glucose level of the alloxan induced severely hyperglycemic rats which were targeted for the 4th phase of blood glucose response of this present study, in line with the study of [17] on the regulation of insulin production by pancreatic beta cells.Observation of both the alloxan induced mild and severe hyperglycemic rats after six hours and fourteen days of treatment with moringa oleifera seed extract of same dose of 400mg/kg body weight in all groups, showed that there was a significant decrease(P<0.05) in the fasting blood glucose level as shown in figures 1 and 2. For the mildly hyperglycemic group, there was a 37.1% decrease in fasting blood glucose level, when the extract was given orally. While a 42.8% decrease in fasting blood glucose level was noted when the extract was given intraperitoneally. Also a 69.7% and 89.6% decreases in fasting blood glucose level were noted when the extract was administered orally and intraperitoneally respectively to the severely hyperglycemic group of rats. The ability of the seed extract of moringa oleifera to significantly reduce hyperglycemia induced by alloxan may be as a result of its phytochemical and micronutrient constituents. A major phytochemical constituent of the extract that have been reported is flavonoids, which has been further characterized by structure and functional relationships as; flavans, flavanones, flavones, flavanols, flavanonols, cetechins, anthocyanidins and isoflavones. Bioflavonoids are well known for their multi-directional biological activities including hypoglycemic effects [13]. Also the moringa oleifera contain many powerful antioxidant phytochemicals, especially quercetin and kaempferol. Kaempferol has been shown to have hypoglycemic activities [18, 19]. Also, the mechanisms of actions could be either by increasing the tissue utilization of glucose [31], by inhibiting hepatic gluconeogenesis or absorption of glucose into the muscles and adipose tissues [32].
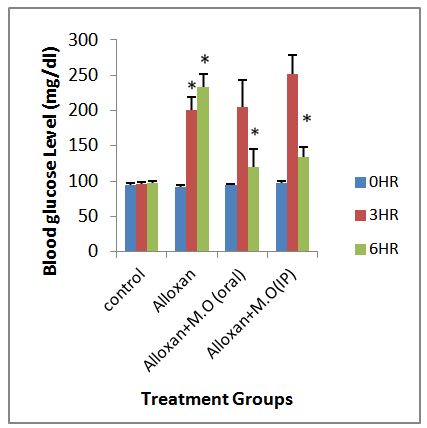 | Figure 1. Effect of Moringa Oleifera Seed Extract on Glucose Level of Alloxan Induced Mild Hyperglycemia |
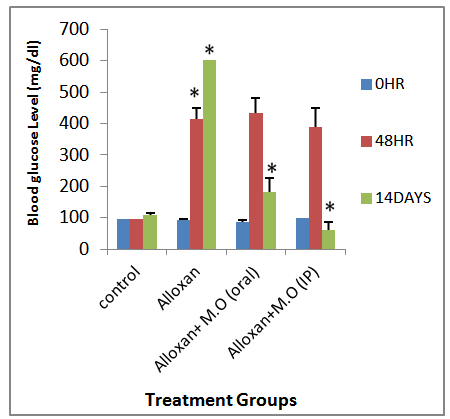 | Figure 2. A sample line graph using colors which contrast well both on screen and on a black-and-white hard copy |
5. Conclusions
- The result of this study has shown that Moringa oleifera seed extract exhibited a hypoglycemic effect on both the mild and severe alloxan induced hyperglycemic rats, indicating that it can be used as a curative plant for the treatment of hyperglycemia and diabetes as well. We therefore recommend that Moringa Oleifera seed be taken as functional foods or nutraceuticals as it is readily available and widely grown.
ACKNOWLEDGEMENTS
- We acknowledge the contributions of technologists: Mr. Okpanachi Emmanuel Egburu and Mr Joseph Egene, from the Department of Physiology, Bingham University, Karu, Nasarawa State, Nigeria.
References
| [1] | Fuglie L J. The miracle tree: The multiple attributes of Moringa. Church World Service, West Africa Regional Office 2001. |
| [2] | Wutythamawech W. Encyclopedia of Thai Herbs, OS Printing, Thailand, 1997. |
| [3] | Mishra G, Singh P, Verma R, Kumar S, Srivastav S, Jha KK, Khosa RL. Traditional uses, phytochemistry and pharmacological properties of Moringa oleifera plant: 2011 An overview. DerPharmacia Lettre 3:141-164. |
| [4] | Aslam, M., Anwar, F., Nadeem, R., Rashid, U., Kazi, T.G., and Nadeem, M.. Mineral composition of Moringa oleifera leaves and pods from dfferent regions of Punjab, Pakistan. 2005 Asian J. PlantSci. 4, 417–421. |
| [5] | Amaglo, N. K., Bennett, R. N., LoCurto, R. B., Rosa, E. A. S., LoTurco, V., Giuffrid, A., LoCurto, A., Crea, F., and Timpo, G.M. Profiling selected phytochemicals and nutrients in different tissues of the multipurpose tree Moringaoleifera L., grown in Ghana. 2010 Food Chem. 122, 1047–1054. |
| [6] | Kurma S. R., Mishra S. H. Anti-inflammatory and hepatoprotective activities of fruits of Moringa Pterygosperma Gaerth. Ind. J. Nat. Prod. 1998; 14:3-10. |
| [7] | Talhiliani P., Kar A. Role of Moringa oleifera leaf extract in the regulation of thyroid hormone status in adult male and female rats. Pharmacol. Res. 2000; 41: 319-323. |
| [8] | Kar A., Choudhary B.K., Bandyopadhyay N. G. Comparative evaluation of Hypoglycemic activity of some Indian medicinal plants in alloxan diabetic rats. 2003 J. Ethnopharmacol.; 84:105-108. |
| [9] | Qaseem A, Humphrey L.L, Chou R. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. 2011 Ann Intern Med. 2011; 154(4):260-267. |
| [10] | Park B.H, Rho H.W Park J.W, Cho C.G, Kim J.S, Chung H.T and Kim H.R. Protective mechanism of glucose against alloxan induced pancreatic beta-cell damage. 1995 Biochem. Biophys Res Commun: 210:1-6. |
| [11] | Alberti, K.G. and Zimmet, P.Z., Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. 1998 Diabet. Med. 15, 539–553. |
| [12] | Giugliano D. Marfella R., Coppola. Vascular effects of acute hyperglycemia in humans are reversed by L-arginine. Evidence for reduced availability of nitric oxide during hyperglycemia. 1997 Circulation 95 (7); 1783-1790. |
| [13] | Szkudelski T. The mechanism of action of Alloxan and streptozocin action in beta cells of the rat pancreas. 2001 Physiol. Res. 50. 536-546. |
| [14] | McLetchie NGB. Alloxan diabetes: the sorcer and his apprentice. 1982 Diabetologia 23. 72-75. |
| [15] | Lenzen S, Tiedge, Jorns A, and Munday R. Alloxan derivative as a tool for the elucidation of mechanism of the diabetogenic action of alloxan. 1996 In: Shafrir E. Lessons from Animal Diabetes.113-122. |
| [16] | Butler. L.K. Regulation of blood glucose level in normal and diabetic rats. Pages 181-202 in tested studies for laboratory teaching, volume 16. (C.A Goldman editor). Proceedings of the 16th workshop/comnference of the association for biology laboratory education. 1995 (ABLE). 273 pages. |
| [17] | Thomas M.K, Rastalsky N, Lee J.H, Habener, J.F. Hedgehog signaling regulation of insulin production of pancreatic beta-cells. 2000 Diabetes vol. 49. No.12 2039-2047. |
| [18] | Fuglie L.J. The miracle tree: Moringa Oleifera: Natural nutrition for the tropics. Church world service, Dakar. pp 68. 1999 Revised in 2001 and published as The Miracle tree: The multiple attribute of Moringa, pp 172. |
| [19] | Ampa Luangpiom, Watchara Kourjampa and Tanaree Junaimaung. Anti-hyperglycemic Properties of Moringa oleifera Lam. Aqueous Leaf Extract in Normal and Mildly Diabetic Mice. 2013 British Journal of Pharmacology and Toxicology 4(3): 106-109. |
| [20] | Toutain, P.L and Bousquet-Melou, A. Bioavailability and its accessment. 2004. J. Vet. Pharmacol. Therap. 27, 455-466. |
| [21] | Yi Zhu, Jaime D’Agostino and Qing-Yu Zhang Role of intestinal cytochrome P450 (P450) in modulating the bioavailability of oral Lovastatin: insite from studies on intestinal epithelium-specific P450 reductase knock out mouse. American society for pharmacology and experimental therapeutics. 2013. Vol. 39 no 6 939-943. |
| [22] | Gaurav Bajaj and Yoon Yeo. Drug delivery system for intraperitoneal therapy. 2010 Pharm Res. 27(5): 735-738. |
| [23] | Aruna R.V, Ramesh, B and kartha V.N.R. Effects of betacarotene on protein glycosylation in alloxan induced diabetic rats. Indian Journal of Experimental Biology.1999 Vol.37, no. 4, pp 399-401. |
| [24] | Lenzen S. the mechanism of Alloxan and streptozocin –induced diabetes. Diabetologia 2008; 51:261-26. |
| [25] | Goldner M.G, Gomori G. studies on the mechanism of Alloxan diabetes. Endocrinology 1944; 35:241-8. |
| [26] | Mythili M.D, Vyas R. Akila G. Gunasekaran S. effects of streptozotocin on the ultra structure of rat pancreatic islets. Microsc Res Tech 2004; 63: 274-81. |
| [27] | Adedapo A.A, Mogbojuri O.M and Emikpe O. safety evaluations of the aqueous extracts of the leaves of moringa oleifera in rats. Journal of medicinal plant research, 2009. vol 3, no. 8 pp 586-591. |
| [28] | Souravh Bais, Guru Sewak Singh and Ramica Sharma. Antiobesity and Hypolipidemic activity of Moringa oleifera leaves agaist high fat diet induced obesity in rats. Advanves in Biology. 2014. Vol 2014. Article ID 162914, 9 Pages. http;//dx.doi.org/10.1155/2014/162914. |
| [29] | Organisation for Economic Co-operation and Development (OECD),Test No. 423: Acute Oral toxicity - Acute Toxic Class Method, OECD Guidelines for the Testing of Chemicals, OECD Publishing, 2002, Section 4, doi: 10.1787/9789264071001. |
| [30] | Oyedepo1 T. A, Babarinde S. O. and Ajayeoba T. A. Evaluation of Anti-hyperlipidemic Effect of Aqueous Leaves Extract of Moringa oleifera in Alloxan Induced Diabetic Rats International Journal of Biochemistry Research & Review 3(3): 162-170, 2013 SCIENCE DOMAIN international www.sciencedomain.org. |
| [31] | Gray AM, Abdel-Wahab YH, Flatt PR. The traditional plant treatment, Sabucus nigra (Elder) exhibits insulin like and insulin releasing actions in vitro. Journal of Nutrition. 2000; 130:15–20. |
| [32] | Kamanyi A, Djamen D, Nkeh B. Hypoglycemic properties of the aqueous root extractsof Morinda lucida (Rubiaceae) study in the mouse. Phytotherapy Research.1994;8:369–371. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML