-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2014; 3(2): 26-29
doi:10.5923/j.medicine.20140302.02
Anti-hepatitis C virus (HCV) Antibody Detection among Fresh Undergraduate Students in Port Harcourt, Nigeria
Okonko I. O. 1, Ikunga C. V. 1, Anugweje K. C. 2, Okerentugba P. O. 1
1Department of Microbiology, University of Port Harcourt, Choba, East-West Road, P.M.B. 5323, Choba, Port Harcourt, Rivers State, Nigeria
2Department of Health Services, University of Port Harcourt, Choba, East-West Road, P.M.B. 5323, Choba, Port Harcourt, Rivers State, Nigeria
Correspondence to: Okonko I. O. , Department of Microbiology, University of Port Harcourt, Choba, East-West Road, P.M.B. 5323, Choba, Port Harcourt, Rivers State, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
This study reports carried out to detect the presence or absence of anti-HCV antibody among fresh undergraduate students in Port Harcourt, Nigeria. The subjects included in this study were 200 [(100(50%)] males and [(100(50%)] females; of ages between 16–20 years, 21–25 years, and 26 years and above. Plasma samples from the subjects were tested for the presence of antibodies to HCV using Global® HCV-Ab kit (Global Diagnostics, USA)and ACON HCV-Ab kit (ACON Laboratories, Inc. San Diego, USA) in a stepwise order for the detection of anti-HCV antibodies. The overall prevalence rate was 0.0%. Also age groups 16–20 years, 21–25 years, and 26 years and above had the same prevalence rate of 0.0%. The prevalence rate of HCV antibody was the same among males and their female counterparts with the rate of 0.0%. This study however, revealed the absence of this infection among the fresh undergraduate students population in Port Harcourt, Nigeria. Further studies on a larger scale is advocated for the generation of data and the use of rapid test kit should be used in conjunction with other immunoassay particularly Elisa technique are therefore recommended.
Keywords: Antibodies, HCV, Undergraduate students, Nigeria
Cite this paper: Okonko I. O. , Ikunga C. V. , Anugweje K. C. , Okerentugba P. O. , Anti-hepatitis C virus (HCV) Antibody Detection among Fresh Undergraduate Students in Port Harcourt, Nigeria, Basic Sciences of Medicine , Vol. 3 No. 2, 2014, pp. 26-29. doi: 10.5923/j.medicine.20140302.02.
Article Outline
1. Introduction
- Hepatitis is an inflammatory condition of the liver while viral hepatitis is a conventional term used to denote hepatitis caused by the hepatotrophic viruses (hepatitis A-G) [1].After its discovery and characterization by Choo et al [2], hepatitis C Virus has remained a major cause of chronic liver disease worldwide and the main reason for liver transplantation in the western world [3]. HCV infections are known to occur in the general population.Approximately 170 million people worldwide who are about 3-4% of the world population are chronically infected with the hepatitis C virus who are at risk of developing liver cirrhosis, cancer or both has been shown to have a worldwide distribution, occurring among persons of all ages, genders, races and regions of the world [4]. Slightly different prevalence was reported from different regions of the world. Prevalence of 1.7% was reported from America, 1.03% from Europe, 3.9% from the Western Pacific, 4.6% from the Eastern Mediterranean, 2.15% from South Asia and 5.3% from Africa [5-7].Among HCV infected persons only 20-30% has symptoms of acute hepatitis. About 75%-85% of infected older adults and 50-60% of infected Juveniles or young adults become chronically infected. Majority of persons with chronic HCV infections are asymptomatic [8].Currently, new HCV infections are primarily due to intravenous or nasal drug use, and to a lesser degree to unsafe medical or surgical procedures. Parenteral transmission via tattooing or acupuncture with unsafe materials is also implicated in occasional transmissions. The risk of perinatal and of heterosexual transmission is low, while recent data indicate that promiscuous male homosexual activity is related to HCV infection [9].The factors that determine the outcome and natural course of HCV infection are not completely understood. However, it is generally accepted that next to virological factors innate and adaptive immune responses play an important role in both, control of HCV infection and as disease pathogenesis [10]. HCV replicates mainly in hepatocytes, but its nucleic acids have also been found in peripheral blood mononuclear cells and in central nervous system cells [11].The detection of anti-HCV antibodies in plasma or serum is based on serological tests especially the use of enzyme immunoassays (EIAs) or Enzyme Linked Immunosorbent assays (ELISA) which are now commercially available[6] and molecular (PCR being used to determine the extent of the virus variation) [12]. This study reports carried out to detect the presence or absence of anti-HCV antibody among fresh undergraduate students in Port Harcourt, Nigeria.
2. Materials and Methods
2.1. Study Area
- The study area is O.B. Lulu-Briggs Medical Health Centre Abuja Campus, University of Port Harcourt Choba, located at the municipal area of Port Harcourt. Port Harcourt is the capital city of Rivers State, also known as the Garden City is located in the forest zone of south southern Nigeria. Port Harcourt city lies on the longitude 7° East of Greenwich meridians and latitude 4°75' North of the Equator.Port Harcourt's heaviest precipitation occurs during September with an average of 370 mm of rain. December on average is the driest month of the year; with an average rainfall of 20 mm. Temperatures throughout the year in the city is relatively constant, showing little variation throughout the course of the year. Average temperatures are typically between 25°C-28°C in the city [13,14]. The city is an important trade majorly petroleum and educational centre and houses one of the largest and foremost teaching hospitals in Africa.
2.2. Study Population
- After obtaining due permission from the Health Center management and informed consent was obtained from fresh students after thorough explanation of the study, 200 blood samples were collected from apparently healthy fresh undergraduate students undergoing medical examination at the O.B. Lulu Briggs University Health Center, University of Port Harcourt, Port Harcourt, Nigeria. Students’ demographic variables considered as risks factors for contracting HCV were collected by the administration of questionnaire forms.
2.3. Specimen Collection and Processing
- About 5ml blood sample was aseptically collected by venipuncture from each student into anticoagulant blood sample bottles. The blood samples were left to clot, after which the plasma was then pipette into sterile Eppendorf tubes and stored by at -20℃ until ready for use.
2.4. Assays for Detection of Hcv Antibody
- Parallel tests were carried out to detect anti-HCV antibodies in the plasma using Global® HCV-Ab kit (manufactured by Global Diagnostics, USA) and ACON HCV-Ab kit (ACON Laboratories, Inc. San Diego, USA). These kits were used in a stepwise order for the detection of HCV antibody in the blood. This method which is immuno-chromatographic and qualitative in nature, detect the presence of HCV antibody in human blood and can be read in-vitro having more than 99.9% sensitivity and 98.6% specificity. The test and interpretation of the results were done in accordance with the guidelines of the Kit’s manufacturers.
2.5. Statistical Analysis
- Here we present data generated with descriptive statistics. Statistical associations or a lack thereof between participant variables and prevalence rates of dual infection were determined using binary logistic regression analysis to estimate odds ratios (OR) with 95% confidence intervals (CI). A p value < 0.05 was set as statistical significance. The analysis was performed with SPSS 15.0 for Windows (SPSS Inc., Chicago, IL).
3. Results Analysis
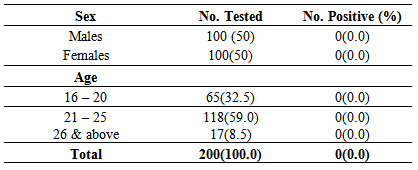
- A total of 200 male and female samples were tested in equal proportions and showed 0.0% prevalence and detection of HCV antibody according to age groups also showed 0.0% prevalence. Equal proportions of 200 male and female samples were tested for HCV antibodies and they both showed the same prevalence rate of 0.0% as shown in Table 1.In the age group 16-20 years, a total of 65 samples were tested out of which none tested positive thus, giving the prevalence of 0.0%. Age groups 21-25 years and 26 years and above showed prevalence of 0.0% and 0.0% respectively as shown in Table 1.
|
4. Discussion
- In this study, we established the zero prevalence of anti-HCV antibody in 200(100 males and 100 females) fresh undergraduate students undergoing medical examination at the O.B. Lulu Briggs Health Center (University of Port Harcourt Medical Center). Since hepatitis C virus is an important cause of morbidity and mortality, it is necessary to carry out studies that provide basic information about the virus in the University of Port Harcourt, Nigeria.The prevalence rate of 0.0% obtained in this study was lower compared to similar study among fresh undergraduate student of University of Ilorin which was 8.0% by Udeze et al [15]. This figure shows a lower rate compared to 4.3% reported by Ojule et al [16] among pregnant women on Port Harcourt. This is far lower compared to the 12.3% HCV prevalence rate reported by Halim and Ajayi [17]. In addition, the prevalence of 0.0% obtained in this study is also lower than the 8.4% HCV prevalence reported from Abuja by Agwale et al [18]. Ayolabi etal [1] reported a sero-prevalence rate of HCV among blood donors in Lagos, Nigeria to be 8.4%, which is also higher than the one observed in the present study (0.0%).A prevalence of 2.3% was reported in Markudi, North Central Nigeria by Achinge et al [3], 3.4% in Kano as reported by Bala et al [19]. In Yola, 2.4% were positive for HBsAg and anti- HCV as reported by Olokoba et al [20].The prevalence of 0.0% obtained in this study is comparable to the 0.0% HCV seroprevalence reported by Elfaki et al [21] in Sudan; and the 0.0% HCV seroprevalence reported by Alli et al. [22] in Ibadan, Oyo State, Nigeria. It is also compared slightly to the 0.2% found in the work of Abdalla et al [23]; the 0.4% documented by Imoru et al. [24] in Kano State, North central Nigeria; the 0.5% in the work of Ejele et al [25]; the 0.5% reported in previous studies by Okonko et al. [26] in Ibadan, Oyo State, Nigeria and the 0.85% reported by Chandra et al. [27] in India.This rate (0.0%) is lower than the 3.0% worldwide seroprevalence reported by the World Health Organization (WHO) in 1999 [28]; the 1.5% previously reported by Okonko et al. [7] in Abeokuta, Ogun State, Nigeria; and the 1.0% reported in previous studies by Okonko et al. [6] in Ibadan, Oyo State, Nigeria. Different prevalence was reported from different regions of the world. Prevalence of 1.7% was reported from America, 1.03% from Europe, 3.9% from the Western Pacific, 4.6% from the Eastern Mediterranean, 2.15% from South Asia and 5.3% from Africa [28]. These variations could be due the fact that prevalence of HCV greatly differs according to the geographical location of a population.
5. Conclusions
- From the results obtained in the present study, it can be clearly stated that hepatitis C virus infection do not exists among the study population. Although with the level of enlightenment on sexually transmitted infections (STIs) and transfusion transmitted infections (TTIs) in Nigeria, no investigation has been carried out on HCV infection rate among students of University of Port Harcourt, Port Harcourt, Nigeria. Thus, the prevalence recorded in this study cannot be compared with past years as no data was present. Moreso, this study was done using rapid test in which according to Adeyemi et al [29] is not sensitive enough to confirm hepatitis statue. Other studies elsewhere have indicated a significant prevalence rate in other population.There is need for larger prospective studies on the prevalence and chronicity of hepatitis C virus in students within the country. Even though serological test remains a valuable tool for the identification of etiology of HCV infection, we recommend that, rapid test kit should be used in conjunction with other immunoassay particularly ELISA technique. There is a need to develop and implement a comprehensive strategy to prevent and control this infection. On the overall, there should be public enlightenment about the presence of HCV infection and possible risk factors.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML