-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2014; 3(2): 17-25
doi:10.5923/j.medicine.20140302.01
Does Ginger Extract Protect against Ethylene Glycol Induced Hepatic Toxicity in Adult Male Albino Rats?
Naser A. ElSawy1, 2, Eman Mohamed Faruk3, Ragia M. Hegazy4, 5
1Department of Anatomy and Embryology, Faculty of Medicine, Zagazig University, ARE
2Department of Laboratory Medicine, Faculty of Applied Medical Sciences, UQU, KSA
3Histology and Cell Biology Department, Benha Faculty of Medicine, ARE
4Department of Pharmacology and Toxicology, Faculty of Pharmacy, UQU, KSA
5Department of Forensic Medicine & Clinical Toxicology, Benha Faculty of Medicine, ARE
Correspondence to: Eman Mohamed Faruk, Histology and Cell Biology Department, Benha Faculty of Medicine, ARE.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Background: Ethylene glycol (EG)is a colourless, odourless, sweet-tasting chemical mainly used as antifreeze which is fatal if ingested. Ginger is used as spices and as an herbal medicine (antioxidant) in Asian countries. Aim of the work: was to evaluate the protective role of Ginger against the Ethylene glycol hepato-toxicity in rats. Material and Methods: Thirty rats were divided into three equal groups: Group I (control group): GIa; 5 rats received saline and GIb; 5 rats received ginger (dose as in GIII), Group ΙΙ: were intraperitoneal injected by EG 0.75 mL for 2 consecutive days then orally administered via intra-gastric tube by EG in a daily dose of 0.1 mL /kg ethylene glycol Group ΙΙI: received EG injection with 1 mL of Ginger extract (24 mg/mL) three times weekly for 6 weeks. Blood samples were collected and livers were microscopically examined. Results: EG induced significant reduction (P=002) in Rats' BW in G II with 30% MR in comparison with GI and GIII. AST, ALT, ALK P, TBIL, and globulin levels in G II were significantly elevated (P=0.02); meanwhile there were significant decrease (P=0.03) in total protein, albumin, and A/G ratio. Microscopic examination showed: increase fibrous tissue and cellular infiltration around the portal tract in G II. Positive antioxidant effect of Ginger over the EG toxicity in G III by apparent decrease of fibrosis, cellular infiltration, vacuolation and necrosis of hepatocytes. Some hepatic lobules regained their normal architecture with proliferated bile ductules. Conclusions: Ginger might be more effective in amelioration of ethylene glycol induced hepato-toxicity.
Keywords: Ginger, Ethylene Glycol, Toxicity, Blood, Liver of Rats
Cite this paper: Naser A. ElSawy, Eman Mohamed Faruk, Ragia M. Hegazy, Does Ginger Extract Protect against Ethylene Glycol Induced Hepatic Toxicity in Adult Male Albino Rats?, Basic Sciences of Medicine , Vol. 3 No. 2, 2014, pp. 17-25. doi: 10.5923/j.medicine.20140302.01.
Article Outline
1. Introduction
- Ethylene glycol (EG), an important organic solvent used in paints, printing inks, and thinners. It causes significant hematologic and central nervous system disturbances following occupational exposure [1]. The toxicity of lower molecular weight ethylene glycol ethers and their acetates haven been extensively studied and found to have hematological, neurological, reproductive and teratogenic toxicity in both animal and human subjects [2-4], But little is known about hepato-toxicity of Ethylene glycol. EG related hepato-toxicity studies on human subjects were mainly case reports only dealing with accidental poisoning. Such reports provided few, if any, information about hepatic effects of Ethylene glycol [5-7]. Simultaneous damage of liver and kidneys in acute poisoning occurs very often in other studies [8, 9]. EG may be absorbed by inhalation and through the skin and ingestion. It may cause serious poisoning [10].The liver and kidneys are primary site of metabolism for ethylene glycol. it is first oxidised by alcohol dehydrogenase to glycoaldehyde, which is then further metabolised to glycolic acid by mitochondrial aldehyde dehydrogenase and cytosolic aldehyde oxidase. Glycolic acid is then metabolised to glyoxylic acid by glycolic acid oxidase or lactate dehydrogenase. Glycolic acid oxidase also catalyses the formation of oxalic acid from glyoxylic acid [11].Side pathways include the conversion via intermediary metabolism of glyoxylic acid to malate, formate and glycine. Glyoxylate may also induce lactic acid formation via oxalomalate production and its inhibitory effect on the citric acid cycle. It is the accumulation of these acid products that accounts for much of the toxicity of ethylene glycol; glycolic acid is the most abundant metabolite of ethylene glycol [11].Ginger was described by Labania (2005) [12] as a plant belongs to Zingiberaea and its scientific name is Zingiberofficinale. Ginger has many uses to treat; sickness of pregnant women [13], sexual weakness, the involuntary urination in children, catches cold, and nervous tension [12].Ajith et al. (2007) [14] suggest that the hepatoprotective effect of Ginger against acetaminophen – induced acute toxicity is mediated either by prevent the decline of hepatic antioxidant status or due to its direct radical scavenging capacity. Tao et al. (2008) [15] found that Ginger had definitive cytoprotective actions in rat hepatocytes exposed to oxidative damage. Antioxidant activity of Ginger due to several compounds such as gingerol, gingerdiol, and gingerdione [12]. This study aimed to evaluate the protective role of Ginger against the Ethylene glycol hepato-toxicity in rats.
2. Materials and Methods
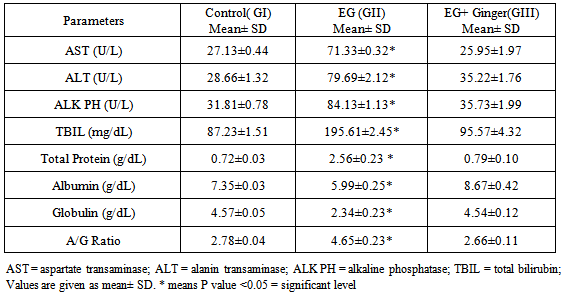
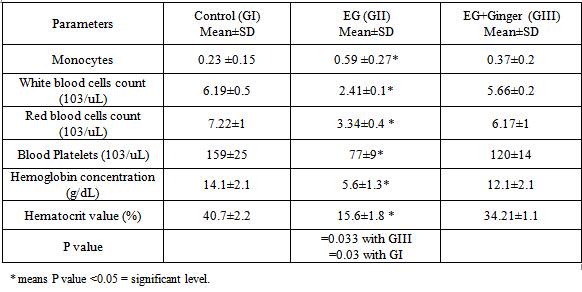
- DrugsThe drugs used were as follows: Ethylene glycol: Purchased from Sigma Chemical Company (St. Louis, Missouri, USA) [16].Preparation of Ginger aqueous extractGinger (Z. officinale Roscoe) rhizomes were purchased from the local market of herbs marketat Benha, Egypt. One kilogram fresh Ginger rhizome was cleaned, washed under running tap water, cut into small pieces, air dried and powdered. 125 g of this powder were macerated in 1000 mL of distilled water for 12 h at room temperature and were then filtered. The filtration was carried out using a muslin cloth and then through Whatman qualitative grade 1 filter paper. This procedure was repeated twice and then all the filtrates obtained were combined and concentrated on a rotary evaporator (RE-111, Buchi, Flawil, Switzerland) accompanied with B-700 recirculation chiller and a water bath model 461 at 65°C under vacum. Finally, the extract change to a thick pasty mass called as crude extract, yielding approximately 12% which reconstituted in distilled water (100 mg/mL) to be used in the study. The concentration of the extract is 24 mg/mL. Each animal in the present study was orally given 1 mL of the final aqueous extract; the dose of Ginger was selected according to Sakret. al. (2011) [17].Animal GroupingThirty rats (195-235 g) were obtained from the animal house, Moshtohor faculty of Veterinary Medicine, Benha University, and received balanced diet with free access to water. All animal procedures were performed according to approved protocols and in accordance with the recommendations for the proper care and use of laboratory animals. Thirty adult male albino rats were randomly selected and three equal groups: Group I (control group): GIa; 5 rats received saline and GIb; 5 rats received Ginger1 mL of final aqueous extract of Ginger(24 mg/mL) three times weekly for 6 weeks. Group ΙΙ: received 0.75mL EG intraperitoneal injection twice with one day interval then in drinking water EG (0.1 mL /kg).Group ΙΙI: received 0.75% EG injection with 1 mL of Ginger extract (24 mg/mL) three times weekly for 6 weeks.Preparation of Ginger aqueous extractGinger (Z. officinale Roscoe) rhizomes were purchased from the local market of herbs at Benha, Egypt. One kilogram fresh Ginger rhizome was cleaned, washed under running tap water, cut into small pieces, air dried and powdered. 125 g of this powder were macerated in 1000 mL of distilled water for 12 h at room temperature and were then filtered. The filtration was carried out using a muslin cloth and then through Whatman qualitative grade 1 filter paper. This procedure was repeated twice and then all the filtrates obtained were combined and concentrated on a rotary evaporator (RE-111, Buchi, Flawil, Switzerland) accompanied with B-700 recirculation chiller and a water bath model 461 at 65°C under vacuum. Finally, the extract change to a thick pasty mass called as crude extract, yielding approximately 12% which reconstituted in distilled water (100 mg/mL) to be used in the study. The concentration of the extract is 24 mg/mL. Each animal in the present study was orally given 1 mL of the final aqueous extract; the dose of Ginger was selected according to Sakret. al. (2011) [17].Experimental parametersTwenty-four hours after the end of the experimental period the animals were weighted, anaesthetised and sacrificed using ether anaesthesia.1. Biochemical examination: Blood was collected by heart puncture and the serum was separated by centrifugation for biochemical assay.1.a. Liver function tests: the serum was extracted from each blood sample for spectrophotometric determination levels of gamma-glutamyltransferase (GGT) [18], aspartate transaminase (AST) and alanine transaminase (ALT) [18], alkaline phosphatase (ALP) [18], total bilirubin [19], total protein, and albumin [20]. Also, the globulin values and Albumin/globulin ratios were calculated.1.b. Hematological Assays: The heparinised blood samples were employed for measurement of red blood cell (RBC) count, white blood cell (WBC) count, platelet (PLT) count, hemoglobin (Hb) concentration and hematocrit percentage (Ht%) by using an automated hematologyanalyzing system.Histological and histochemical studiesLiver sections were prepared for histological study (H&E) and Masson’s trichrom stains) study and Electron microscopic examination. Each excised liver was divided into two parts. One part was fixed in formalin 10%, prepared for paraffin sections of 4-6 µm thickness and stained with hematoxylin and eosin for histological structure and massoۥs trichrome stains to demonstrate the collagen fibers for light microscopic examination [21]. The second part (1mm x 1mm thickness) was fixed in glutaraldehyde for 20 hours then washed with phosphate buffer, fixed in 1% tetraoxide. Ultrathin sections were cut by an ultramicrotome then stained with uranyle acetate and carried on copper grids [22]. These procedures were performed at the regional centre for mycosis and its applications, Al-Azhar University.StatisticsAll quantitative data were presented as mean (X) ± Standard Deviation (SD) and were compared using SPSS version 17.0 software (SPSS, Chicago, Illinois, USA). P value <0.05 was considered statistically significant.
3. Results
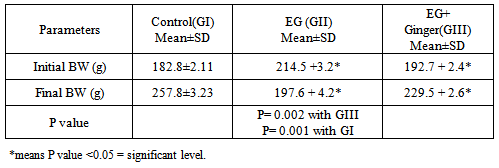
- The toxic EG injection in (G II) resulted in 30% mortality rate (3/10) in rats over the study period. However, when crude extract of Ginger was concomitantly administered with EG, it fully protected the rats from the acute lethal effect of EG and no mortality rate was recorded in G III.The recorded results of the body weight of administration of EG affect the growth of rats as evidenced by highly significant decrease (P= 0.002; P= 0.001 respectively) in their final rats' body weight in comparison with GI, and GIII. In untreated toxic rats (GII), the initial body weights ranged 199-232 gm with a mean of 214.5 +3.2 gm while the final body weight ranged from 180 to 215 gm with a mean of 197.6 + 4.2 gm. In treated toxic group (GIII), the initial body weights ranged from 200 to 225 gm with a mean of 192.7 + 2.4 gm while the final body weights ranged from 218 to 243 gm with a mean of 229.5 + 2.6 gm shown in Table 1.
|
|
|
4. Histological Results
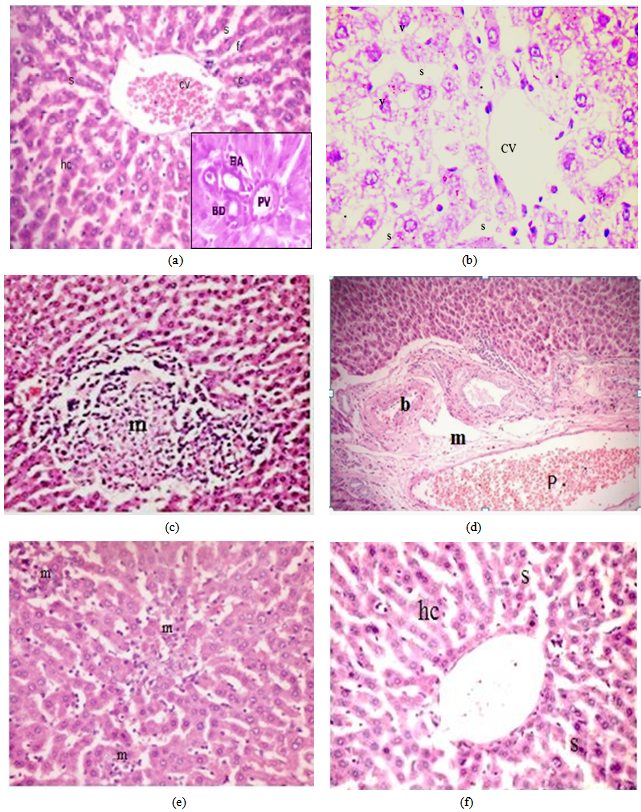
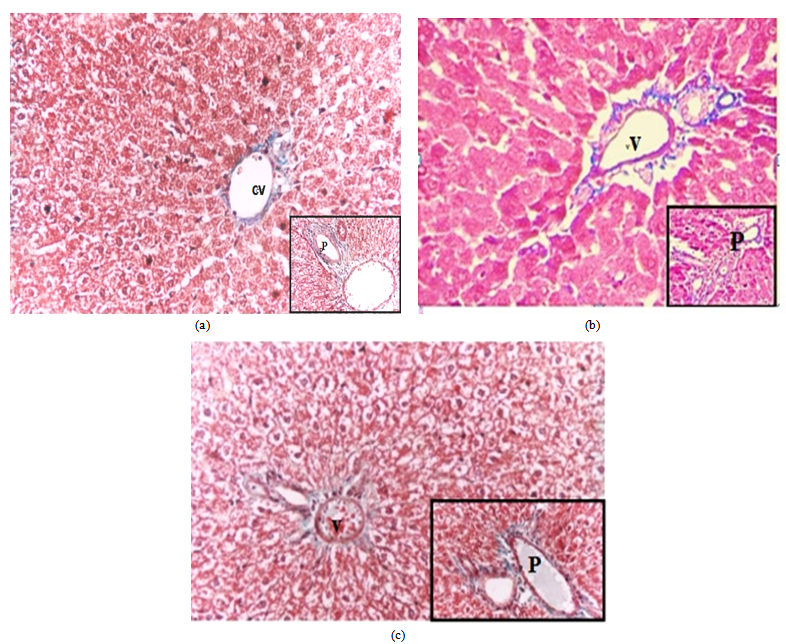
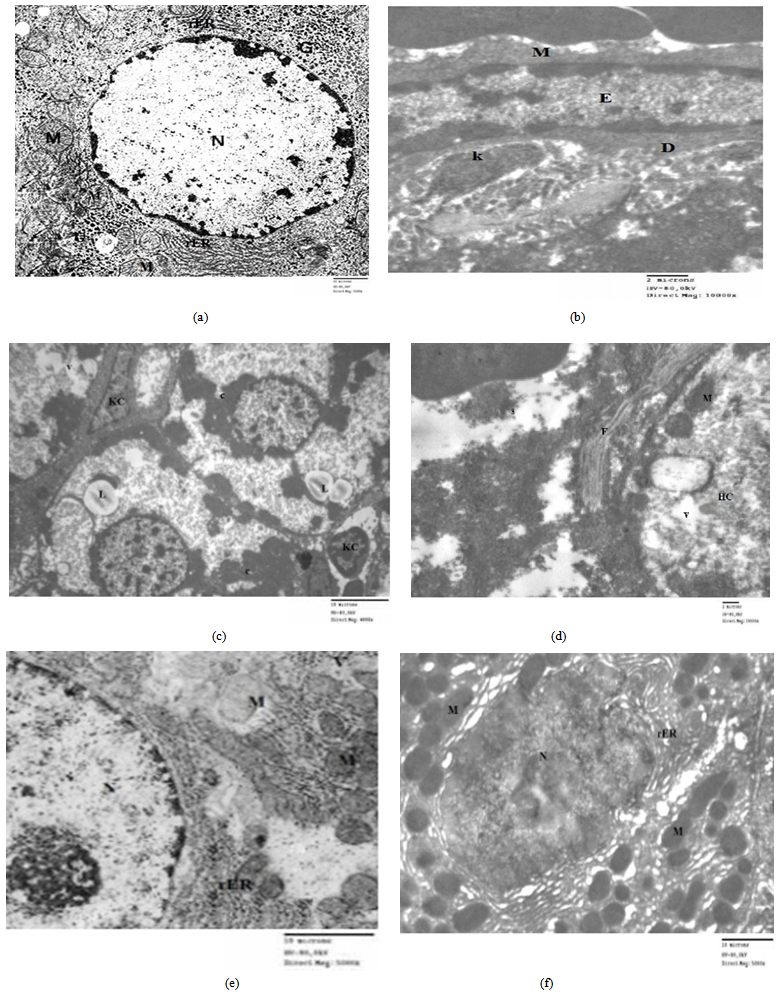
- Light microscope results:H&E stain: The liver sections stained with H&E in control group (Fig 1a) showed normal hepatic lobules consist of branching hepatocytes sheets radiating from central vein to the periphery of the lobules and separated by blood sinusoids. The portal spaces contain branch of hepatic artery and portal vein as well as bile ductules. However, liver sections from EG group (GII) showed different histological changes in their hepatic architectures such as degeneration and necrosis of some hepatocytes and mononuclear inflammatory cell infiltrations in the liver parenchyma. The cytoplasm of hepatocytes was vacuolated. Liver cords and cells were widely separated from each other with loss of the hepatic architecture and dilatation of the blood sinusoids with dilated congested central vein (Figs1, b-c-d). Group III (EG + Ginger) showed decrease fibrous tissue and cellular infiltration around the bile ductule in preexisting portal tract with less or normal hepatic architecture. Normal cords of hepatocytes with smaller cytoplasmic vacuoles separated by blood sinusoids (Figs1, e-f).Masson's trichrome stain: The liver showed few collagen fibres around the central vein in control liver sections (Fig 2,a). However, examination of liver sections from EG group (GII) (Fig 2,b) showed severe to moderate collagen fibres around the central vein and at portal areas compared to the control group.Group III (EG+Ginger) showed decrease collagen fibers around the central vein and at portal areas (Fig 3,c).Electron Microscopic ExaminationLiver sections from control group (GI) showed normalultra structural appearance of the hepatic cells with ground cytoplasm. Rough endoplasmic reticulum and ribosomes were detected. Mitochondria were rounded or oval and normal appearance of blood sinusoid with endothelial lining and Kupffer cell in the Disse's space (Fig 3a, b).The liver tissues from EG (GII) group affected hepatocytes were separated by large rarefied areas indicate extensive necrotic areas with vacuolization of the hepatocytes cytoplasm, sinusoidal endothelial damage, irregular nucleus with disturbed and electrolucent nucleoplasm, degenerated mitochondria, mitochondrial swelling, Ito cell changed to myofibroblast with collagen fibre, swollen rough endoplasmic reticulum, intense glycogen accumulation or rosettes, damaged Ito cells, deformed Disse's space and dilated veins (Figs 3c, d).Group III (EG+ Ginger) showed decreased organelle injury with rERs appeared to be normal with normal nucleus, and mitochondrial cristae were much more visible, less vacuolation of hepatocyte cytoplasm (Figs 3e, f).
5. Discussion
- The usefulness of ethylene glycol (EG) and theirs acetates derivatives can be attributed to their physicalproperties, particularly their miscibility or high solubility in water and other organic solvents, and their lowvapour pressures. Most of ethylene glycol (EG) is used in coating industries [23, 24]. In the present study was designated to document the toxic effects of exposure to ethylene glycol on rat livers and possible protective role of Ginger. Administration of EG led to extreme weight loss and ultimately death of the rats with a significant increase of the body weight of the livers in the Ginger treated group. Ginger antioxidant effect against chemical induced hepatotoxicity had been approved with previous study [25, 26]. Also, antioxidant efficient effect in reversal of alcohols especially EG hepatotoxicity [26, 27].Abnormalities in liver function indices with alcohols had been reported in the current study were in accordance with other studies that recorded a progressive elevation in ALT, AST, GGT enzymes, and protein concentrations.Abnormal laboratory results may also be obtained owing to an altered pathophysiology or enzyme induction secondary to EG-associated liver toxicity [28, 29]. However there is significant correction of liver function with an increase in the body weight in Ginger treated group in the present study. In agreement with these results: Afshin et al, 2012 Samira, 2013 and Gunathilake, et al. 2014 [30-32]; who found that Ginger antioxidant and protective role against organ toxicity.Among our interesting findings in this study are the hematological results, whereas there was a remarkable decrease in the number of WBCs, RBCs, and blood platelets in the blood of rats with ethylene glycol overdoses; and also they were associated with significant decreases in total hemoglobin and hematocrit that associated also with increase of monocytes count. This was in agreement with Starek, et al. 2012 [33]. However, treatment of these rats with crude extract of Ginger significantly prevents these hematological toxic effects of ethylene glycol as proven by Samira, 2013 [31]. Aye, et. al; 2010 and Diab et. al; 2012 [34, 35] in harmony with our findings, a study recorded the same fact in mice and the authors found that many chemicals as ethylene glycol in its overdose liberates some of the toxic products that have powerful DNA destructing effects on bone marrow DNA and so decrease of all blood elements .Histopathological examination of liver of quails treated with ethylene glycol revealed severe inflammation and degenerative changes in hepatocytic characterized by swelling in hepatic cells degenerative changes increased congestion of blood vessels, cell necroses was also observed. Moreover, inflammatory leucocytic infiltration hypertrophied and hyperchromatic, pyknotic and enlarged nuclei were evident. The present findings are in consequence with the results of the previous investigations [34, 35].An apparent increase in collagen fibers around the central vein and the portal areas was seen in the present study. This was in accordance with other studies, which showed that an increased amount of fibrous stroma was an early sign of liver toxicity [34, 35]. Moreover, collagen fibers and large stellate cells were seen in the sinusoidal spaces of the liver in the present study. In chronic inflammation or cirrhosis the stellate cells play an important role in hepatic fibrogenesis by the deposition of type I and III collagen within the sinusoidal space [34, 36].Concerning the effect of Ginger, present study indicated that Ginger improved the histological and biochemical alterations induced by EG in liver of rats. These observations support concept of many researches who found that Ginger can be used to enhance liver functions and amelioration of hepatic toxicity [25, 26, 31, 32, & 37]. Also, agree with (Nakazawa and Ohsawa; 2002) [38] who mentioned that hepatic enzymes of mice have an important role in 6-gingerol metabolism. Also the present results are in accordance with [39] who recommended Ginger to be used to reduce the serious effect of esteritin on the histological structure of albino mice liver, where liver was able to resume the normal structure. Those authors considered hepatocellular damage to be from a reduction in ATP supply. Hamza; 2010 [40] had elucidated that hepatocellular damage could be correlated with the disrupted enzymes activities.The toxicity of EG may also be due to the difference of underlying metabolic pathway which involves an alkylation reaction, producing the formaldehyde and aldehyde and ethylene glycol. Ethylene glycol can be further metabolized to glycolate by alcohol/aldehyde dehydrogenase enzymes [38, 41, and 42].The main pharmacological actions of Ginger and compounds isolated there from include immuno-modulatory, anti-tumorigenic, anti-inflammatory, anti-apoptotic, anti-hyperglycemic, anti-lipidemic and anti-emetic actions. Ginger is a strong anti-oxidant substance and may either mitigate or prevent generation of free radicals. It is considered a safe herbal medicine with only few and insignificant adverse/side effects [43]. Oxidants and antioxidants have attracted widespread interest in nutrition research, biology and medicine. It has become clear that constant generation of pro-oxidants, including oxygen free radicals, is an essential attribute of aerobic life [37, 44]. A disturbance in the pro-oxidant/antioxidant system has been defined as oxidative stress. Reactive oxygen species (ROS) are very reactive molecules ranked as free radicals owing to the presence of one unpaired electron such as a superoxide ion (O2.), nitrogen oxide (NO) and hydroxyl radical (HO.). Even though naturally present in the organism, they are mainly confined to cell compartments and counterbalanced by natural antioxidant molecules, such as glutathione, glutathione peroxidase, superoxide dismutase, vitamin E and vitamin C, acting as free radical scavengers [43, 45]. Ginger extracts have been extensively studied for a broad range of biological activities, especially antioxidant activities [37] found that Ginger significantly lowered lipid per oxidation by maintaining the activities of the antioxidant enzymes –super oxide dismutase, catalase and glutathione peroxides in rats. Also in acute inflammation, there is an increase in polymophonuclear cells which lead to increases in local production of H2O2, and ultimately to large amounts of ROS and cellular damage. Decreasing ROS will lead to better resolution of acute inflammation. The major active phenolic ingredients isolated from Z. officinale (Zingerone, Gingerdiol, Zingibrene, gingerols and shogaols) have antioxidant activity [44-46].It is concluded from the present study that the protective effect of Ginger extract against hepatotoxicity induced by ethylene glycol may be attributed to its antioxidant properties.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML