-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2013; 2(1): 14-20
doi:10.5923/j.medicine.20130201.03
Classification, Causes and Clinical Implications of Sacral Spina Bifida Occulta in Indians
Rajani Singh
Department of Anatomy, AIIMS, Rishikesh, 249201, India
Correspondence to: Rajani Singh, Department of Anatomy, AIIMS, Rishikesh, 249201, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Precise and systematic knowledge of normal and classified variant anatomy of sacral spina bifida occulta is clinically important for anesthetists in locating sacral hiatus for nerve blocking, neurologists for bladder and bowl disorders, radiologists for correct interpretation of images, orthopedic surgeons for surgical procedures and academically to anatomists for correlating classes, their genesis and clinical implications. Therefore the study has been carried out. The examination of 140 dry sacra resulted in 55 normal and remaining 85 with sacral spina bifida occulta. The sacra with occult spina bifida were morphologically classified in five categories. This congenital defect was genetically analyzed based on the results derived from mice and clinical significance was elucidated. Classified sacral spina bifida occulta were as follows-Type-1(1.4%) with completely open sacral canal, Type-II (2.1%) below S1 down to S5, Type-III (22.1%) below S2 down to S5, Type IV (33.6%) below S3 down to S5 and Type-V S1 (1.4%) to S2 open with normal sacral hiatus at the apex of sacrum. This study will not only enrich the encyclopedia of anatomy with new information on classification but also provide correlation between classification, genesis and clinical implications for the first time. The knowledge will be of paramount importance to anatomists and clinicians.
Keywords: Bowl, Bladder, Genesis, Morphology, Sacral Canal
Cite this paper: Rajani Singh, Classification, Causes and Clinical Implications of Sacral Spina Bifida Occulta in Indians, Basic Sciences of Medicine , Vol. 2 No. 1, 2013, pp. 14-20. doi: 10.5923/j.medicine.20130201.03.
Article Outline
1. Introduction
- Spina bifida is a developmental defect of the vertebral column in which laminae do not fuse and spinal cord remains relatively unprotected because of absence of bony dorsal wall on spinal cord. Spina bifida may be divided in two types depending upon its exposed or occult condition:1. In spina bifida cystica, if meninges alone herniate out through bifida, it is known as meningocele or if both meninges and neural tissue protrude out through this defect, this is known as myelomeningocele[1]. 2. In spina bifida occulta, the meninges and/or neural tissue remain underneath skin. This defect is indicated more often by a skin lesion such as a hairy patch, dermal sinus tract, dimple, hemangioma, or lipoma .The occurrence of spina bifida occulta in sacral region spreading from S1 to S5 has been termed as sacral spina bifida occulta (SSBO). Non-closure of laminae to various levels of sacrum constitutes SSBO[2, 3]. Many sacra have S5 or also S4 open, exposing the dorsal surface of the fifth sacral body[4]. Rare and complete open sacral wall has also been observed as cases[5, 6]. Radiological studies have investigated the prevalence of this condition in the sacrum[7] in various populations, also analyzing X-rays that were originally taken for other diagnostic purposes[8] or combined X-ray and computed tomography to determine the level of SBO-S1[9] and the radiographic study of 53 cadavers validated by six dissected cadavers from Australian population reported the incidence of variant SSBO[10]. Total occult sacral dysraphism from S1 to S5 (dorsal interlaminar dehiscence), forming a channel between the laminae has been reported by Vasilica et al.,[11]. Sacral spina bifida occulta has been recorded as a congenital anomaly by Kumar and Tubbs[12]. Several studies of this defect have been carried out by many authors[13, 14, 15] on the name of sacral hiatus to improve the failure rate in caudal epidural block. The SSBO and associated diseases, including posterior disc herniation, backache, enuresis and neurological abnormalities of the feet, and functional disorders of the lower urinary tract[10, 9, 16, 17] are very common. This enhances the importance of the present study. The classification of SSBO carried out under this study will not only facilitate the clinicians in systematic diagnosis and treatment of above mentioned diseases but also the anatomists will be provided with enhanced data base and systematization of study of SSBO. Additionally, the genetic causes have also been analysed in this piece of work for exercising appropriate precautions to control diseases related to this defect detected during developmental period or after, through cautious treatment and genetic counselling. Therefore the study is of paramount importance for clinicians, radiologists and anatomists.
2. Material and Methods
- 140 dry Indian sacra obtained from the human Osteology laboratory, Department of anatomy, have been examined for variant anatomy of sacral spina bifida occulta. The varied morphology of defective sacra has been classified into five categories of SSBO depending upon the longitudinal extent of opening of dorsal wall/sacral canal. The morphological measurement of maximum width of body along with ala of S1, length and width between sacral cornua of open part of sacral canal and the dimensions of non-fused gaps between vertebral bodies corresponding to these classes have been measured by digital vernier calipers. The measurements have been taken thrice by the same person to avoid inter observer error. Causes of SSBO have been analyzed genetically depending on the experiments on mice.
3. Results
- The broken including sacralized sacra have been excluded from the study. The classification of various types of SSBO is illustrated below- SSBO in all figures stands for sacral spina bifida occulta. 1. Type-I SSBO (Fig.1D and Fig. 1V): Dorsal wall is completely open starting from top of S1 spine continuing up to sacral hiatus at tip of sacrum in this class of SSBO.
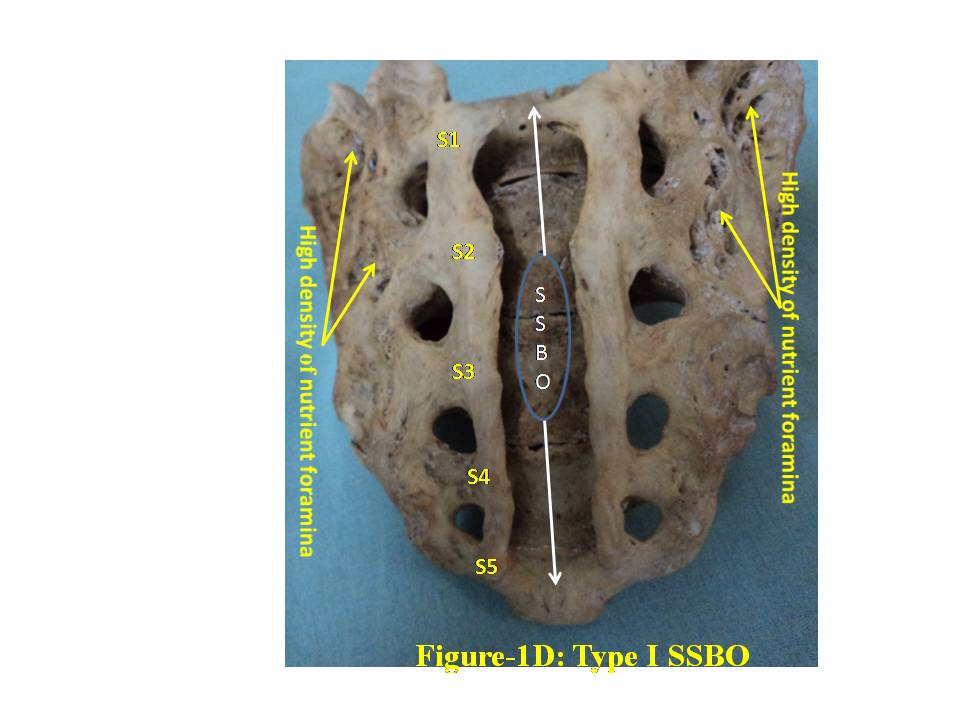 | Figure 1D. represent Type-I bifida on dorsal surface of sacrum. S1, S2, S3, S4 and S5 show sacral spine1 to spine 5, SSBO stands for sacral spina bifida occulta |
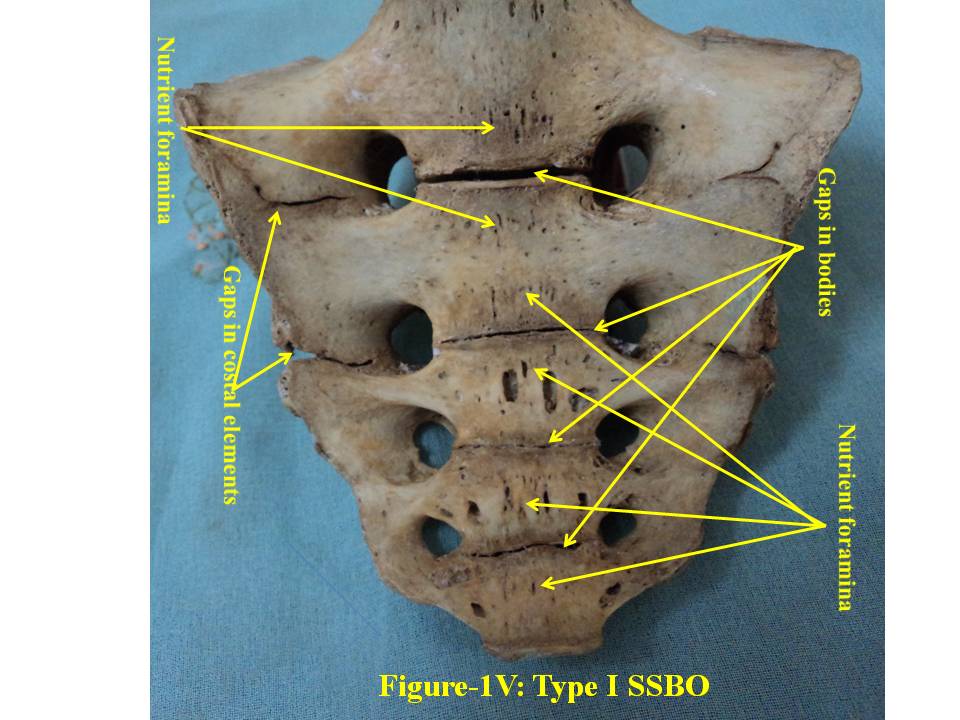 | Figure 1V. presents ventral surface of Type-I bifida. SSBO stands for sacral spina bifida occulta |
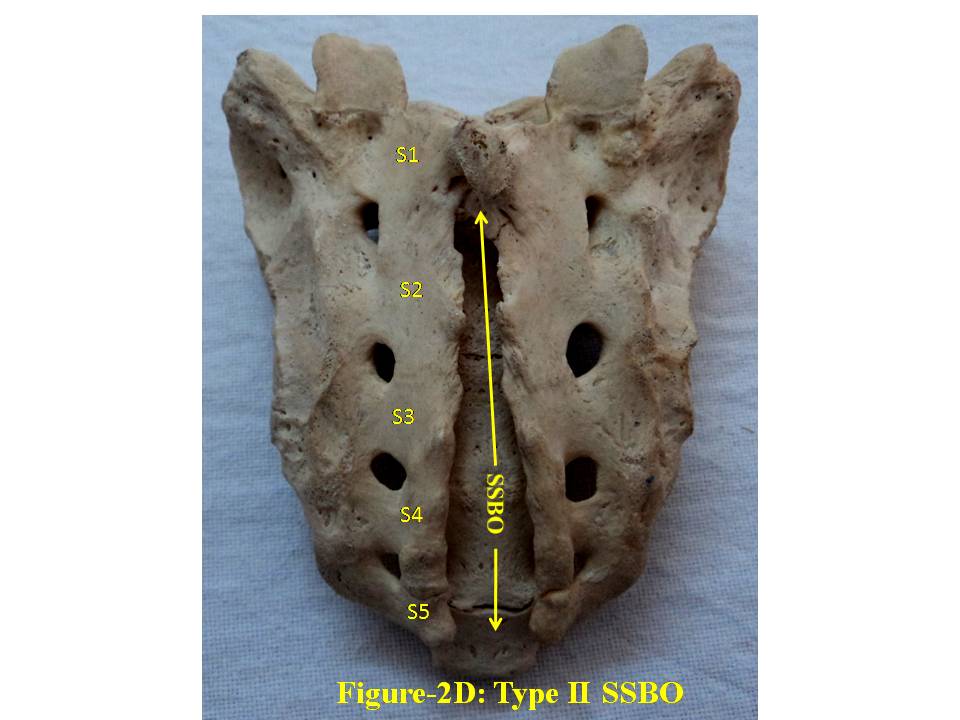 | Figure 2D. shows Type-II bifida on dorsal surface of sacrum. S1, S2, S3, S4 and S5 show sacral spine1 to spine 5, SSBO stands for sacral spina bifida occulta |
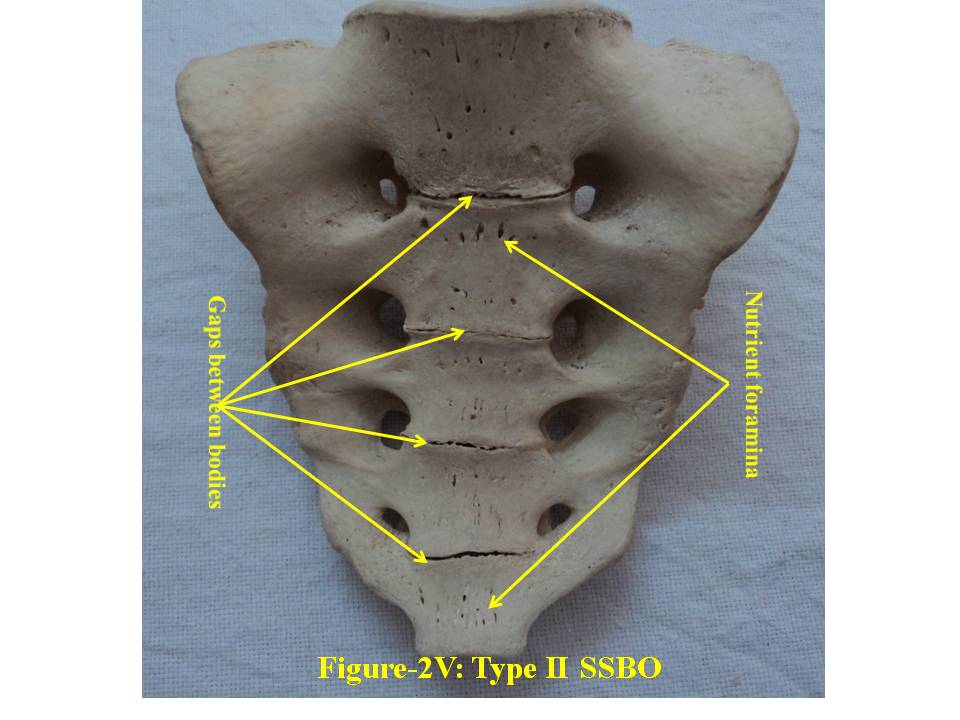 | Figure 2V. presents ventral surface of Type-II bifida. SSBO stands for sacral spina bifida occulta |
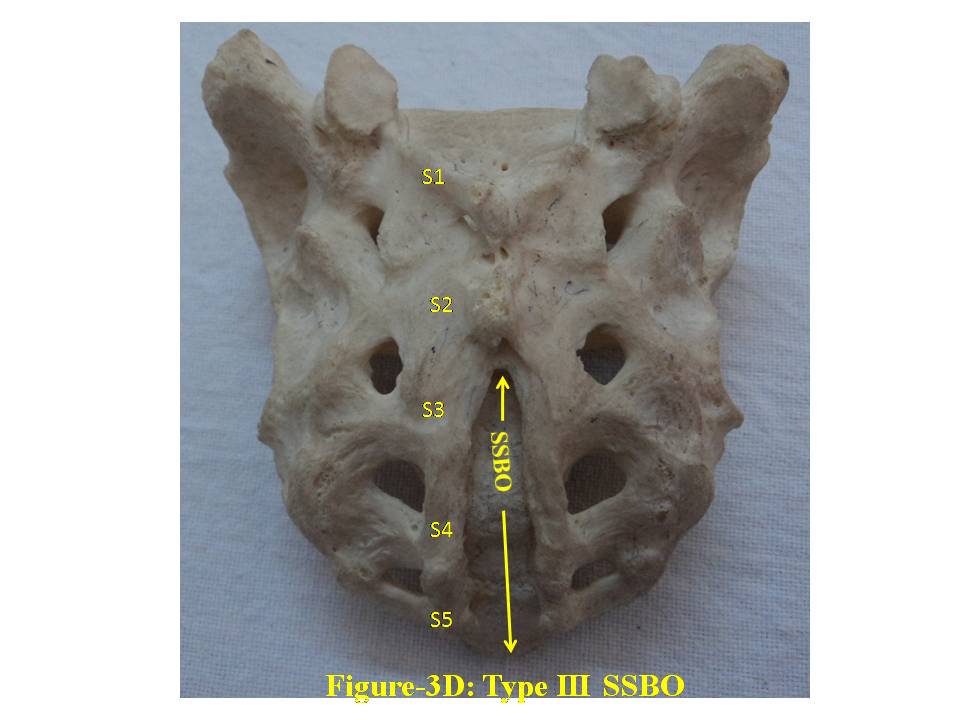 | Figure 3D. shows Type-III bifida on dorsal surface of sacrum. S1, S2, S3, S4 and S5 show sacral spine1 to spine 5, SSBO stands for sacral spina bifida occulta |
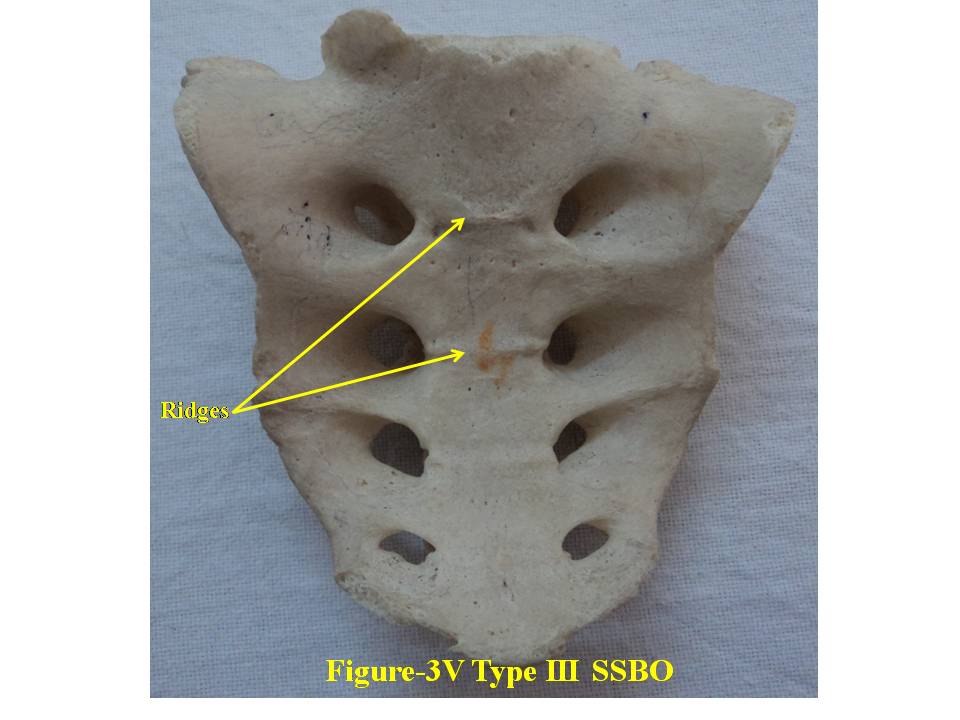 | Figure 3V. presents ventral surface of Type-III bifida. SSBO stands for sacral spina bifida occulta |
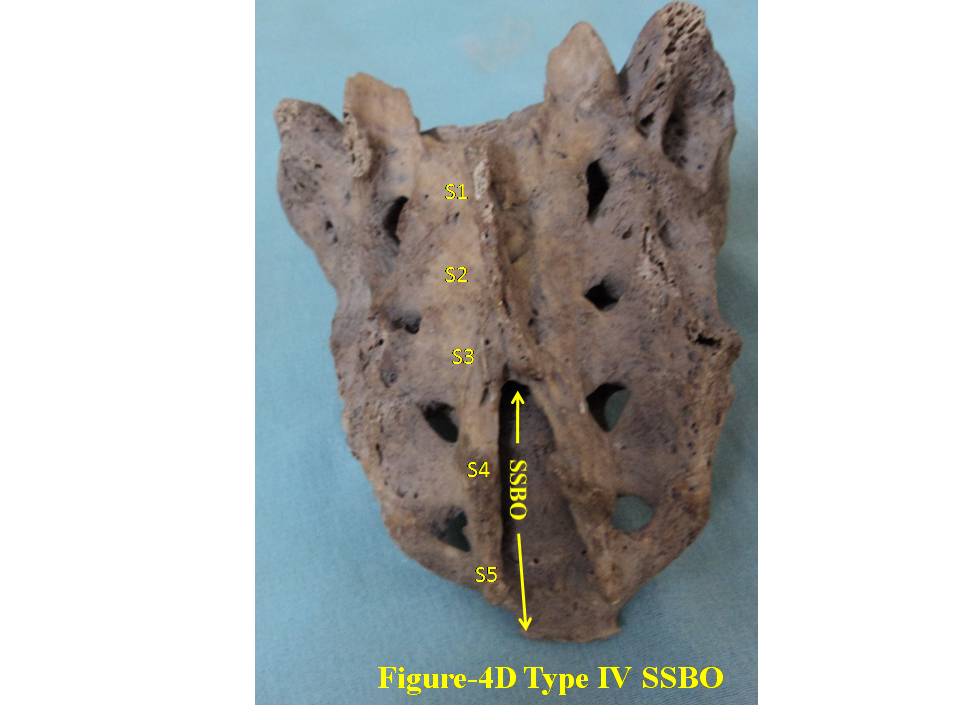 | Figure 4D. shows Type-IV bifida on dorsal surface of sacrum. S1, S2, S3, S4 and S5 show sacral spine1 to spine 5, SSBO stands for sacral spina bifida occulta |
 | Figure 4V. presents ventral surface of Type-IV bifida. SSBO stands for sacral spina bifida occulta |
|
 | Figure 5D. shows Type-V bifida on dorsal surface of sacrum. S1, S2, S3, S4 and S5 show sacral spine1 to spine 5, SSBO stands for sacral spina bifida occulta |
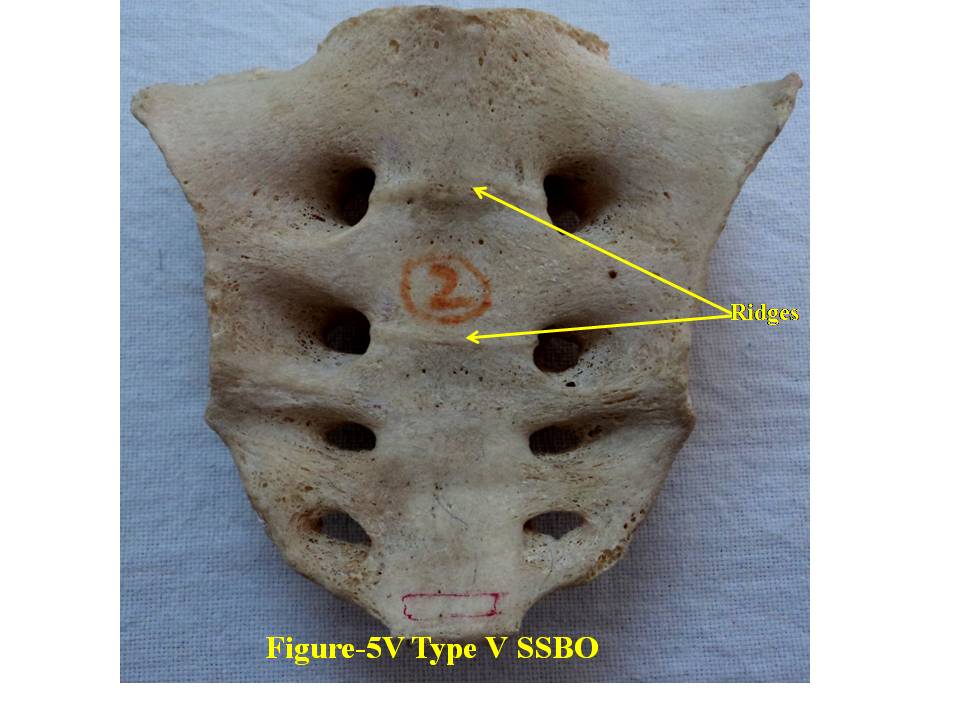 | Figure 5V. presents ventral surface of Type-V bifida. SSBO stands for sacral spina bifida occulta |
4. Discussion
- For comprehensive and systematic study of the entire spectrum of sacral spina bifida occulta (SSBO) as observed in this experiment has been classified in five classes as elaborated in result section. Since spinal cord ends at L1 level and the dural sac ends at the level of the second vertebrae of the sacrum but total spina bifida and detection of the dura mater just beneath the hiatus have also been reported in 1% of cases[18], therefore sacral canal (in a normal case) below this level contains extradural fat, vertebral venous plexus, lower sacral nerve roots and the filum terminale only. As present study is focused on the variant anatomy of open sacral canal, so in case of classified SSBO’s Type-I, II and V, Dural sac, extramural fat, sacral nerve roots and film terminable are exposed to skin remaining underneath it. Under this new envelop of soft skin in place of hard dorsal wall the covered structures may generate signs of presence of SSBO over the skin. Thus a skin lesion such as a hairy patch, dermal sinus tract, dimple, hemangioma, or lipoma may be indicators of underlying SSBO. Additionally, the lesions indicating herniation of lipomatous mass may attach to nerve roots thus tethering them. Lipomatous mass envelops both dorsal and ventral nerve roots, or only the dorsal nerve roots, or simply the filum terminale. These lesions have a more guarded prognosis than simple meningoceles. Apart from this, morphometry of the SSBO/sacrum shows many variations in the dimensions of maximum width of body, ala, length and width of open part of sacral canal between the two sacral cornua, the dimensions of non-fused gaps between vertebral bodies and occurrence of ridges on the ventral side of sacrum (Table-1). As the sacral canal is completely open (Type-I) and open below S1 (Type-II), the sacrum becomes weak and likely to be fractured by even minor trauma.One important observation is that in Type-I and Type-II SSBO’s, the bodies are not completely fused. This generated varying degree of non-fused gaps between the adjacent sacral vertebrae. These gaps are associated with high density of vascular foramina. These gaps besides decreasing from cranial to caudal end of the sacrum due to incomplete fusion are not present in subsequent types of SSBO’s. This shows that more the length of SSBO is, the more defective fusion of bodies of sacral vertebrae has been. Costal element too did not completely fuse in type-I SSBO. The ridges between sacral vertebrae on the ventral side are prominent opposite to the absence of bifida on the dorsal side (type III and type IV) except in type V SSBO where though the bifida is present from S2 and above yet there is a prominent ridge between S1 and S2. Density of vascular foramina is more in Type I and Type II SSBO at the places where the gap between bodies of adjacent vertebrae is more. This may be due to the fact that the wider gap between the bodies of adjacent vertebrae might have reduced the strength of bone. Hence to compensate for weak sacrum, high density vascular foramina might have formed for providing better nutrition to strengthen the sacrum. The mean and standard deviation of length of opening of SSBO has been analysed and tabulated in Table-1.The frequency of occurrence and percentage has statistically been computed and compared (Table-2).
4.1. Genetic Causes of SSBO
- Genesis of sacrum is controlled by expression of Hox11 family of genes as mice with deletion of Hox11 gene completely lack sacrum[19]. Pax1 is expressed in sclerotome cells which are fated to form body and intervertebral disc. Cells migrating dorsally to neural tube which forms neural arches stop expressing Pax1 and start expressing Pax9 gene[20]. Pax1 expression becomes stronger in the ventral medial part of the sclerotome that will form the vertebral body and inter-vertebral disc whereas Pax9 is reinforced in the posterior ventral lateral compartments which are progenitors of the neural arch[21]. The cells migrating dorsally to the neural tube form dorsal part of neural arches and spinous process of the vertebra[20, 22]. Thus this population forms cartilage in close vicinity to the ectoderm despite the inhibitory activity of the ectoderm on chondrogenesis[23, 24, 25]. Sonic Hedgehog (SHH) mutant mice present defective cartilage and epaxial muscle development[26]. Foxc2 is expressed in the entire sclerotome including the cells migrating dorsally to the roof plate. Its expression depends on SHH activity and Foxc2 mutants exhibit defective vertebral bodies and neural arches. Double mutants for Foxc2 and Pax1 present with more severe phenotype including largely open neural arches and absence of vertebral bodies and intervertebral bodies[27]. This phenotype is due to reduction of proliferation and impaired development of the sclerotome[27]. Wnt molecules produced by the ectoderm prevent Pax1 activation[22]. The roof plate or high Bone morphogenetic protein (BMP) activity could specifically be required to release ectoderm inhibition on cartilage differentiation locally[28].
4.2. Genetic Causes in Classified SSBO for Each Class
- In Type-I and Type-II SSBO, incomplete fusion of the vertebral bodies and intervertebral discs may be because of combined modulated effect of under expression of Hox11 and Pax1 gene families, defective secretion of molecules like SHH from neural tube and inactivation of Pax1 by Wnt molecules. As regards completely open dorsal wall, either there is decreased molecule secretion from roof plate or decreased BMP activity. Hence inhibitory activity of Wnt molecules on chondrogenesis produced by ectoderm has not been overcome. Non-fusion of neural arches may be due to double mutation of Pax9 and Foxc2 genes. In type-III SSBO, prominent ridges on ventral surface might be due to over expression of Hox11, Pax1 and high BMP activity. The prominent ridges may be due to over activity of chondrocytes/ osteocytes. Dorsal wall is open below S2 spine which may also be formed due to double mutation of Foxc2 and Pax9 genes. In type-IV SSBO, Open dorsal wall may have been caused by double mutation of Foxc2 and Pax9 genes and low activity of BMP or decreased secretion of molecules by roof plate but there must be normal expression of Hox11 and Pax1genes as vertebral bodies are normally fused. In Type-V SSBO, prominent ridges on ventral surface may be due to over expression of Hox11 and Pax1. Non development of laminae may be due to mutation in the expression of Pax9 as this is required for the formation of neural arches. Either there is impairment of activity of molecules secreted from roof plate or reduced BMP activity which are essential for cartilage formation of neural arches.
4.3. Clinical Significance
- 1. In Type-I, II and V, the sacral canal below L1 level contains extradural fat, vertebral venous plexus, sacral nerve roots and the filum terminale. Meninges may herniate through occult opening between S1 and S2 underneath skin will be injured by even minor trauma. This may cause CSF leakage resulting drop in CSF pressure. Besides this, the sacral nerves will be damaged by external trauma causing weakness in lower limbs, bladder and bowel disorders. Incomplete development of the bones of the dorsal neural arches of the upper sacrum may be a marker of incomplete neurogenesis of the sacral nerves[17].This causes incontinence where there can be either partial or complete loss of voluntary urination. Almost all people with spina bifida may have some form of bowel incontinence due to damage to lower sacral nerves[17]. Sacral spina bifida occulta is suggested to be linked with a variety of conditions including posterior disc herniation, backache, enuresis and neurological abnormalities of the feet, and functional disorders of the lower urinary tract[9, 10, 16, 17]. 2. In Type-I and II in addition to above, attachment of erector spinae and multifidus muscles to dorsal surface may be defective in SSBO affecting biomechanical dynamics of vertebral column creating more clinical complications. 3. In Type-I to V, morphology of sacrum is clinically important for the caudal epidural block performed for the diagnosis and treatment of lumbospinal disorders[5, 18]. The knowledge of exact topographical anatomy of the sacrum is essential for locating sacral hiatus for such procedures. An important point in caudal epidural block is awareness of the distance between the sacral hiatus and dural sac, anatomically to avoid dural puncture.4. Presence of sacral spina bifida occulta may increase the chances of damage to sacral nerves and create difficulty in internal fixation of screw[5]. 5. Open sacral canal and associated gaps between vertebral bodies weaken the sacrum and so become more liable to fracture during internal screw fixation and instrumentation or otherwise. Moreover, as the overall strength of the sacral bone due to completely occult sacral bifida reduces so even minor external trauma is likely to cause fracture of sacrum besides other clinical complications. Therefore on the diagnosis Type-I and Type-II SSBO in patients may be alerted by clinicians to remain cautious from external trauma.The classification of SSBO has been carried out by the examination of only 140 (small sample space) dry sacra from India. More work is recommended from widely diversified area on large populations with extended variety of SSBO. The limitation of dry bone study is that it is not carried out on live patients so clinical implications are based on knowledge and experience of theory of medical science and clinical researches carried out. The genetic study is extended from experimental results from mice to human beings along with embryological studies carried out. So some inferences drawn on the basis of this study may be verified by clinicians for its establishment.
5. Conclusions
- The SSBO has been classified in five categories. The causes of SSBO too have been analyzed with a special mention to genetics category-wise. Though the study pertains to dry bones yet the clinical significance has been brought out by clinical cases and known pathological condition due to these classified defects as summarized in table 3.
|
ACKNOWLEDGEMENTS
- The paper is dedicated to my father, Mr. Man Singh for his guidance which enlightened me like a beacon. Dr. Mukesh Singla Associate Professor of AIIMS is gratefully acknowledged for information support. Author gratefully acknowledges the appreciations by visitors of the poster presented in annual meet of AAA with EB2013 at Boston USA on April 22 2013. No external funding could be obtained from any source. There is no conflict of interest.
References
| [1] | Sadler TW. Langman’s Medical Embryology. 11th edn. Philadelphia Lippincot Williams and Wilkins 2009: 302-303. |
| [2] | Brailsford JF. The radiology of bones and joints. 5th edn. London J & A Churchill 1953: 674-690. |
| [3] | Romanes GJ. ed. Cunningham’s Textbook of Anatomy. 12th Ed. Oxford Oxford University Press: 1981; 626–639. |
| [4] | Williams PL, ed. Gray’s Anatomy. 38th Ed., New York Churchill Livingstone 1995:1828–1831. |
| [5] | Srijit D, Shipra P. Spina bifida with higher position of sacral hiatus: a case report with clinical implications. Bratisl. Lek. Listy 2007; 108: 467–469. |
| [6] | Senoglu Nimet, Senoglu Mehmet, Gumusalan Yakup. International Journal of Anatomical Variations. 2008; 1: 26–27. |
| [7] | V. Macchi, A. Porzionato, C. Stecco, and R. De Caro. Radiologic Anatomy of the Sacral canal. A. Alexandre et al. (eds.), Advances in Minimally Invasive Surgery and Therapy for Spine and Nerves, Acta Neurochirurgica Supplementum 2011; 108: 5-8 |
| [8] | Boone D, Parsons D, Lachmann SM, Sherwood T. Spina bifida occulta: lesion or anomaly? Clin Radiol 1985; 36:159–161. |
| [9] | Avrahami E, Frishman E, Fridman Z, Azor M. Spina bifida occulta of S1 is not an innocent finding. Spine 1994; 19:12–15. |
| [10] | Albrecht TL, Scutter SD, Henneberg M. Radiographic method to assess the prevalence of sacral spina bifida occulta. Clin Anat 2007; 20:170–174. |
| [11] | Vasilica- Monica Graza, Angela Simalcsik and Luminita Bejenarid. Analele Științifice ale Universității. “Alexandru Ioan Cuza” din Iași, s. Biologie animală, Tom LVIII, 2012; 195-204. |
| [12] | Kumar A, Shane Tubbs R. Spina bifida: A diagonostic dilemma in Paleopathology. Clin Anat 2011; 24(1): 19-33. |
| [13] | Vinod kumar, Pandey SN, Bajpai RN, Jain PN, Longia GS. Morphometrical study of sacral hiatus. Journal of Anatomical Society of India 1992; 41 (1): 7-13. |
| [14] | Nagar S K. 2004. A study of Sacral Hiatus in dry human sacra. Journal of Anatomical Society of India 2004; 53 (2) 18-21. |
| [15] | Padeyappaanavar Kiran V, Bhusareddi P S, Harsh M P, Naglikar A S. Agenesis of Dorsal Wall of Sacral Canal. Anatomica Karnataka 2011; 5 (1): 69-71. |
| [16] | Fidas A, MacDonald HL, Elton RA, McInnes A, Wild SR, Chisholm GD. Prevalence of spina bifida occulta in patients with functional disorders of the lower urinary tract and its relation to urodynamic and neurophysiological measurements. BMJ. 1989; 298: 357–359. |
| [17] | Galloway NT, Tainsh J. Minor defects of the sacrum and neurogenic bladder dysfunction. Br. J. Urol. 1985; 57: 154–155. |
| [18] | Sekiguchi M, Yabuki S, Satoh K, Kikuchi S. An anatomic study of the sacral hiatus: a basis for successful caudal epidural block. Clin J Pain. 2004; 20(1):51–54. |
| [19] | Wellick DM and Capecchi M R. Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science 2003; 301: 363-367. |
| [20] | Monsoro-Buro AH and Le Douarin N. Duality of molecular signalling involved in vertebral chondrogenesis. Curr Top Dev Boil 2000; 48: 43-75. |
| [21] | Neubuser A, Koseki H, Balling R. Characterization and developmental expression of pax9, a paired box containing gene related to pax1. Dev Bio 1995; 170: 701-11. |
| [22] | akahashi Y, Monsoro-Buro AN H, Bontoux M and Le Douarin N M. A role for quox-8 in the establishment of the dorsoventral pattern during vertebrate development. Proc Natl Acad Sci USA 1992; 89:10237-41. |
| [23] | Capdevila J, Tabin C, Johnson R L. Control of dorsoventral somite patterning by Wnt-1 and beta-catenin. Dev Biol. 1998; 193: 182-194. |
| [24] | Kenny-Mobbs T, Thorogood P. Autonomy of differentiation in avian branchial somites and the influence of adjacent tissues. Development 1987; 100(3): 449-62. |
| [25] | Marcelle C, Ahlgren S, Bronner- Fraser M. In vivo regulation of somite differentiation and proliferation by sonic hedgehog. Dev Biol 199; 214:277-87. |
| [26] | Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H and Beachy PA. Cyclopia and defective axial patterning in mice lacking sonic hedgehog gene function. Nature 1996; 383: 407-13. |
| [27] | Furumoto TA, Miura N, Akasaka T, Mizutani-Koseki Y, Sudo H, Fukuda K, Maekawa M, Yuasa S, Fu Y, Moriya H, Taniguchi M, Imai K, Dahl E, Balling R, Pavlova M, Gossler A, Koseki H. Notochord-dependent expression of MFH1 and PAX1 cooperates to maintain the proliferation of sclerotome cells during the vertebral column development. Dev Biol. 1999; 210(1):15-29. |
| [28] | Manzanares M, Trainor PA, Ariza-Mcnaughton L, Nonchev S, Krumlauf R. Dorsal patterning defects in the hindbrain, roof plate and skeleton in the dreher (dr1) mouse mutant. Mech Dev 2000; 94:147-56. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML