-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2013; 2(1): 9-13
doi:10.5923/j.medicine.20130201.02
Anticonvulsant Activity of Methanol Leaf Extract of Commiphorakerstingii Engl
F. Khan1, Y. Musa1, A. H Yaro2, N. Yahuza1
1Department of Pharmacology and Therapeutics, Faculty of Pharmaceutical Sciences. Ahmadu Bello University, Zaria-Nigeria.
2Department of Pharmacology, Faculty of Medicine, Bayero University, Kano-Nigeria
Correspondence to: F. Khan, Department of Pharmacology and Therapeutics, Faculty of Pharmaceutical Sciences. Ahmadu Bello University, Zaria-Nigeria..
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
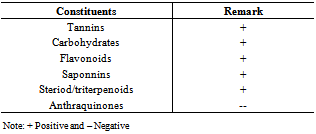
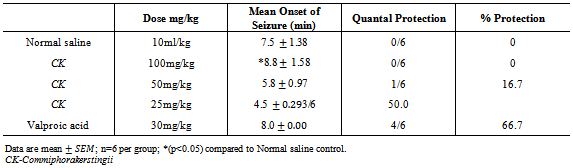
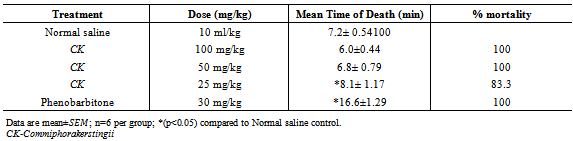
Preparations of Commiphorakerstingii(family-Burceraceae) have been used in African Traditional Medicine for variety of human ailments for many years,including epilepsy. In this study,the anticonvulsant activity of the methanol leaf extract of Commiphorakerstingii was investigated using four animal models at graded doses of 1000, 500 and 250mg/kg. Maximum electroshock test (MEST) in chicks, Pentylenetetrazole(PTZ), Strychnine (STN), 4-Aminopyridine (4-AP)- induced seizures in mice. The extract offered 50% and 16.7% protection against PTZ-induced seizures at 250 mg/kg and 500 mg/kg respectively., Conversely,the extract did not produce a remarkable activity against MEST induced seizure, but protected mice against 4-AP induced seizure by significantly (P < 0.05) prolonging mean time of death significantly at all concentrations tested. The preliminary phytochemical screening revealed the presence of carbohydrates, flavonoids, tannins, saponins, steroids/triterpenes. The intraperitoneal median lethal dose of the extract was found to be 3,809.9mg/kg. These findings suggest that the crude methanol extract may contain bioactive principles with anticonvulsant properties, thus supporting the isolation and development of these bioactive components of the plant as anticonvulsant agents.
Keywords: Commiphorakerstingi,Seizure, Maximal Electroshock, Maximum Electroshock Test (MEST), Pentylenetetrazole (PTZ), Strychnine (STN), 4-Aminopyridine (4-AP)
Cite this paper: F. Khan, Y. Musa, A. H Yaro, N. Yahuza, Anticonvulsant Activity of Methanol Leaf Extract of Commiphorakerstingii Engl, Basic Sciences of Medicine , Vol. 2 No. 1, 2013, pp. 9-13. doi: 10.5923/j.medicine.20130201.02.
Article Outline
1. Introduction
- Medicinal plants have been useful in the development of new drugs and continue to play an invaluable role in drug discovery processes[1, 2]. There is greater reliance on traditional than orthodox medicines by the majority of people in the developing countries, because these traditional medicines are most often in form of herbs or plants, which are abundantly available as such easily assessable, most importantly cheap and trusted by the local people based on their ancestral experience[3]. Commiphorakerstingi (family-Burceraceae) is a tree of 20 -30 feet high with an ever green bark which is smooth and eventually peeling in brownish papery strips, panicles crowned at the end of the branches[4]. It is locally known in Nigeria as ‘Dashi, Ararrabi,Dali, Kwaor, Ganinbul’. Its’ stem bark is used as an antidote in venomous sting bite, in arrow poisoning[4]; has antioxidant and antibacterial[5] and laxative[6] actions, while the leaves have analgesic action[4]. The decoction of the combination of the leaves with other plants is used as a remedy for febrile convulsion in the North western Nigeria (Mohammed Mai Wada, Personal Communication). There is no report in the literature of the anticonvulsant activity of the leaf of the plant. This study, is therefore, aimed at evaluating the anticonvulsant potential of the methanol leaf extract of the plant in laboratory animals.
2. Materials and Method
2.1. Collection and Identification of Plant Material
- The leaf of the plant Commiphorakerstingii was collected in the month of October, 2008 in Samaru village, Sabongari Local Government Area of Kaduna state- Nigeria and authenticated by Mallam Umar Gallah of the Herbarium section of Biological Science Department, Ahmadu Bello University (ABU), Zaria-Nigeria. A voucher specimen was deposited for future reference (Voucher number 006).Preparation of extractThe leaves of the plant were air dried at ambient room temperature. The air dried leaves were then commuted using mortar and pestle to obtain fine power. The powered leaves were defatted using petroleum ether (60-80℃) for 48 hours using soxhlet apparatus. The defatted mark was air dried, thereafter extracted exhaustively with methanol (70% v/v) using soxhlet apparatus. The extract was concentrated on a water bath at 40ºC to a dark green sticky mass subsequently referred to as methanol extract (CK).
2.2. Experimental Animals
- Locally bred Swiss Albino Mice (18-33g) of either sex obtained from Department of Pharmacology and Therapeutics, Ahmadu Bello University, Zaria-Nigeria were used. They were housed in a cage at room temperature, were allowed access to food and water ad libitum and also a day old chicks (25-35g) obtained from National Animal Production and Research Institute (NAPRI), Zaria-Nigeria. All experimental protocols were approved by the university animal ethics committee.
2.3. Preliminary Phytochemical Screening
- Preliminary phytochemical analysis of the methanol extract was carried out to identify the presence of alkaloid, steroid, flavonoid, tannin, reducing sugar, saponin and gum using standard procedures[7].
2.4. Acute Toxicity Study in Mice
- The method of Lorke,[8] was employed to estimate the median lethal dose (LD50) of CK The study was divided into two phases. In the first phase, nine mice were divided into three groups each consisting of three animals and were treated intraperitoneally (with graded doses (1000 mg/kg, 100mg/kg and 10mg/kg) of the CK and observed for signs of toxicity and death over a period of 24 hours; In the second phase of the study, three groups of mice each consisting of one were administered with three more specific doses (5000 mg/kg, 2900 mg/kg, and 1600 mg/kg) of the CK based on the outcome of the first phase. Mortality was recorded within 24hrs and LD50 was estimated as the geometric mean of the highest non-lethal dose in which the animal survived and the lowest lethal dose in which the animal died.
2.5. Maximal Electroshock Test (MEST) in Chicks
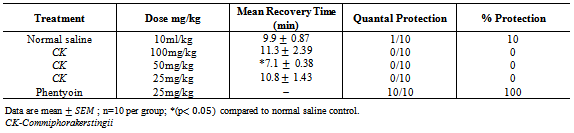
- The method described by Swinyard and Kupferberg[9] was employed. A day old chicks were divided into five (5) groups of ten (10) each. The first group (group I) was administered 10ml/kg of normal salineintraperitoneally. The second, third and fourth groups (Group II, III and IV) were administered 100mg/kg, 50mg/kg and 25mg/kg of the CK respectively. And the fifth group (group V) with phenytoin 20mg/kg, 30 minutes later maximum electroshock was delivered to each chick by stimulus applied via eye-clip electrodes using an ugo-basile electroconvulsive machine. The parameters used were 90mA of current, frequency pulse of 150, pulse width m/s of 6 and shock durations of 0.8. Seizures were manifested as tonic extension of hind limbs, the ability to prevent or shorten the time from this feature was considered as an indication of anticonvulsant activity [10].
2.6. Pentylenetetrazole (PTZ) Induced Seizure in Mice
- The method of Swinyardet al,[10] was employed. 30 mice were divided into five (5) groups of six (6) mice each. Group I was administered normal saline 10ml/kg, Groups II, III, and IV were administered 100mg/kg, 50mg/kg and 25mg/kg of the CK respectively, Group V was administeredsodium valproate 200mg/kg. 30 minutes post treatment mice in all groups were administered PTZ (85mg/kg) subcutaneously and were observed for the presence or absence of convulsion, (tonic spasm, threshold seizure, and loss of right reflexes).
2.7. Strychnine (STN) Induced Seizure in Mice
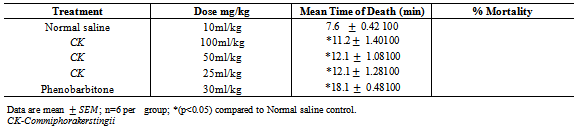
- The method described by Porter[11] was employed. 30 mice were divided into 5 groups of 6 each. Group I was administered normal saline 10ml/kg intraperitoneally. Groups II, III and IV were administered intraperitoneally 100mg/kg, 50mg/kg and 25 mg/kg of the CK respectively. Group V was administered 30mg/kg of phenobarbitone intraperitoneally. 30 minutes post treatment, 2.5mg/kg of STN was administered to all the groups subcutaneously. (The animals were observed for extension jerks of the hind limbs and death within 10min).
2.8. 4 – Aminopyridine (4-A.P) Induced Seizure in Mice
- The method described by Yamaguchi and Rogawski[12] was employed. 30 mice were divided into 5 groups of 6 each. Group Iwas administered normal saline 10ml/kg intraperitoneally. Groups II, III and IV were administered 100m/kg, 50m/kgand 25mg/kg of the CK respectively intraperitoneally. Group V was administered 30mg/kg of phenobarbitone intraperitoneally. 30 minutes post treatment the mice in all the groups were administered with 4-A.P (15mg/kg subcutaneously.). The animals were observed for presence or absence of convulsion (excitation, wild movement, tonic hind limb extension and death) for a period of 30 minutes.
2.9. Statistical Analysis
- Results were expressed as mean ± standard error of mean. The results were analyzed for statistical significance using one-way ANOVA followed by Dunnets’ post hoc t-test for multiple comparisons. A P value less than 0.05 were considered significant.
3. Results
- Preliminary phytochemical screening
|
|
|
|
|
4. Discussion
- The results obtained from this study suggest that CK contains some biologically active compounds that possess some anti-seizure potential. The intraperitoneal median lethal dose of the extract suggests that it is relatively safe[13]. The inability of the extract to protect against Maximal electroshock test suggests that it may not be beneficial in the management of generalized tonic-clonic seizures. Tonic hind limb extension is a common feature of maximal electroshock in rodents[14]. Clinically beneficial antiepileptic drugs in the treatment of generalized tonic clonic and partial seizures such as phenytoin and lamortigine have been found to suppress hind limb tonic extension produced by MES.The moderate activity of the extract against PTZ-induced seizure suggests that it may be beneficial in the management of petit mal epilepsy. Anti-epileptic compounds that raise seizure threshold have been reported to be protective against PTZ-induced seizure[15]. PTZ has been reported to interact with GABA neurotransmission[16] as well as central noradrenergic activity[17]. Drugs such as felbamate which block glutamatergic neurotransmission mediated by N-methyl D-aspartate (NMDA) receptors have been found to be protective against PTZ-induced seizure. Therefore, the activity of the extract against PTZ-induced seizure may involve GABAergic, noradrenergic and or glutamatergic neurotransmission. Although the extract did not protect animals against 4-aminopyridine-induced seizure, the significant increase in the onset of seizure is an indication of moderate activity. K+ channels play a significant role in controlling all aspect of neuronal excitability, including resting membrane potential, responsiveness to synaptic inputs, frequency adaptation and neurotransmitters release[18], suggesting that the activity of the extract may involve enhancement of K+channel activity.Strychnine is a competitive antagonist of the inhibitory amino acid glycine[19]. The inability of the extract to protect against strychnine-induced seizure suggests that its activity may not involve glycine receptors. Flavonoids and saponins have been previously reported to possess anticonvulsant activity[20, 21] and may therefore be responsible for the anticonvulsant potential of the extract of CK. It may therefore be concluded that the crude extract of CK possess some anticonvulsant activity, supporting the ethnomedical claim of the use of the plant in the management of epilepsy in Nigeria.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML