-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2012; 1(5): 40-45
doi: 10.5923/j.medicine.20120105.04
Molecular Screening of Obesity Candidate Gene Variant Neuropeptide Y Receptor Type 2 (NPY2R) A172T among Malaysian Subjects
Yau-Wei Lee 1, Phee-Phee Chia 2, Yee-How Say 1
1Department of Biomedical Science, Faculty of Science
2Department of Science and Engineering, Centre for Foundation Studies, Universiti Tunku Abdul Rahman (UTAR) Perak Campus, 31900, Kampar, Perak, Malaysia
Correspondence to: Yee-How Say , Department of Biomedical Science, Faculty of Science.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The association of the A172T Neuropeptide Y Receptor Type 2 (NPY2R) gene with obesity and metabolic syndrome has not been widely studied in the Malaysian population. Therefore, the objective of this study was to investigate the association of NPY2R A172T gene variant with obesity, metabolic syndrome, related anthropometric measurements, glucose level, blood pressure and the demographic characteristics among Kampar Health Clinic subjects. In total, 168 subjects (68 males, 100 females; 66 obese, 102 non obese; 47 Malay, 89 Chinese, 32 Indians) were recruited in this study via convenience sampling and anthropometric measurements were taken. Genotyping was performed using HaeIII Polymerase Chain Reaction-Restriction Fragment Length Polymorphism (PCR-RFLP) revealing 121 AA, 37 AT and 10 TT subjects; minor allele frequency 0.170. NPY2R A172T genotypes and alleles did not show any association with obesity (p = 0.982; 0.857, respectively), gender (p = 0.129; 0.245, respectively) and ethnicity (p = 0.581; 0.390, respectively). Besides, they did not show any association with the presence of metabolic syndrome according to 3 criteria in the modified NCEP ATP III (p = 0.447; 0.288, respectively). In conclusion, the NPY2R A172T gene variant was not associated with obesity, obesity-related traits and metabolic syndrome among Malaysian subjects in this study.
Keywords: Neuropeptide Y Receptor 2, Single Nucleotide Polymorphism, Obesity, Metabolic Syndrome, Anthropometric Measurements, Malaysia
Article Outline
1. Introduction
- Obesity is defined as a state of increased adiposity resulting from chronic nutrient excess and it has reached its epidemic levels in modern society. Malaysia is part of this epidemic too, whereby the prevalence rate of obesity has increased from 4.4% to 14.0% as reported by the Malaysian National Health and Morbidity Survey (NHMS) II in 1996 and NHMS III in 2006, respectively[1]. The increased prevalence of obesity has been accompanied by a parallel increase in the prevalence of the metabolic syndrome (MetS) or “syndrome X”[2]. The definition in diagnosing MetS was first introduced by WHO in 1998 whereby this definition focused on insulin resistance as a main criterion[3]. In addition, the National Cholesterol Education Program Adult Panel Treatment III (NCEP ATP III) and International Diabetes Foundation (IDF) introduced their own set of criteria in diagnosing metabolic syndrome in 2001 and 2005, respectively[4,5]. NCEP ATP III criteria are based on the presence of 3 out of 5 factors in diagnosing metabolic syndrome and the risk factors are waist circumference >102 cm in men and >88cm in women, blood pressure, serum cholesterol, plasma triglycerides and fasting blood glucose[4]. This definition is rather suitable in the US population than Asians due to the low prevalence of metabolic syndrome among Asians[6]. Thus, a modified version of NCEP-ATP III is made in waist circumference for Asians; > 80 cm for women and >90 cm in men is used to define central obesity[7,8]. The neuropeptide Y receptor type 2 (NPY2R) is one of the class of G-protein coupled receptors activated by closely related peptide hormones - neuropeptide Y (NPY), peptide YY (PYY) and pancreatic polypeptide[9]. NPY2R has a heptahelix structure which comprises glycosylation site at the amino-terminal, two extracellular cysteines which form a disulfide bridge and a cysteine molecule at the cytoplasmic tail of the N-linked carbohydrate[10]. It binds selectively to Y2 receptor subtype agonists such as NPY3-36 and PYY3-36[11]. The gene encoding for it, NPY2R, is located on chromosome 4q32.1 which consists 8447 kilobases including 2 exons and the coding region is located on the second exon[12]. Wide expression of NPY2R in the CNS with a particularly high level in an area of the arcuate nucleus (ARC) with a permeable blood brain barrier makes the receptor a potential mediator of peripheral signals in the regulation of energy homeostasis[13] and may be relevant to feeding and body weight control[14]. Activation of the NPY2R reduces appetites while avoiding stimulation of the NPY receptor type 1 (NPY1R) and type 5 (NPY5R) that induces feeding, thus provides a pharmaceutical approach to modulate food intake[14]. The NPY2R gene variant, A172T has a G/A polymorphism predicting non-synonymous base change from Alanine to Threonine base at codon 172 in the coding region of NPY2R exon 2. The heterozygous genotype of this gene variant was first discovered in a 2-year-old Hispanic boy with hyperphagia and severe early-onset obesity in a British study[15]. This gene variant was also subsequently detected among adult Pima Indians subjects; however, was not associated with obesity[16]. Currently, there is limited data and evidence on this association worldwide, especially among the Malaysian population. As different populations show different associations in the existing research, the data on the association of NPY2R A172T variant with obesity in other populations cannot be used to extrapolate for the Malaysian population. Therefore, the objective of this study was to perform genotyping of the NPY2R A172T gene variant among Malaysian subjects from a health clinic in Kampar, Perak to determine the prevalence of the mutated genotypes and alleles, and to investigate if there was any association with obesity. Demographic characteristics, anthropometric measurements, blood pressures and fasting plasma glucose level were also determined to investigate whether there was any association of these obesity and metabolic syndrome-related traits with the NPY2R A172T gene variant.
2. Methodology
2.1. Subjects, Questionnaire and Clinical Measurements
- A total of 168 subjects (mean age: 54.3 ± 14.0; 68 males, 100 females; 47 ethnic Malays, 89 Chinese, 32 Indians; 102 non-obese, 66 obese) were recruited by convenience sampling from the Kampar Health Clinic from June to December, 2011. The exclusion criteria of the subjects include hyperthyroidism, pituitary diseases, chronic liver disease, chronic renal disease, acute infection, haematologic diseases and patients under medications that affect the glucose metabolism. The demographic data included in this questionnaire were age, gender and self-identified ethnicity; while blood pressures and anthropometric measurements consisting of systolic and diastolic blood pressures (SBP, DBP), pulse rate, weight, body mass index (BMI), waist and hip circumferences, total body fat (TBF), subcutaneous fat (SF), visceral fat level (VFL) and skeletal muscle (SM) were taken as described in our previous study[17]. Subjects with the BMI cut-off point of ≥ 27.0 kg/m2 were considered as obese[18]. Overnight fasting peripheral blood drawing was conducted with the aid of a qualified phlebotomist. Fasting plasma glucose level was determined by using OneTouch® Ultra EasyTM blood glucose meter and test strips (LifeScan Inc., CA). The presence of MetS was based on the only three accessible criteria out of five risk factors in accordance to the modified NCEP ATP III definition of metabolic syndrome for Asians: WC > 80 cm for women and > 90 cm in men, hyperglycaemic/having elevated fasting plasma glucose of > 6.1 mM or currently being treated for diabetes, and having blood pressure of ≥ 130/85 mmHg. This study was registered under the National Medical Research Registry NMRR-09-826-4266 and the protocol was approved by the Medical Research and Ethics Committee, Ministry of Health, Malaysia. All individuals that have participated in this study signed informed consent forms and all samples were taken in accordance with the Declaration of Helsinki (revised in Seoul, 2008).
2.2. DNA Extraction and Genotyping
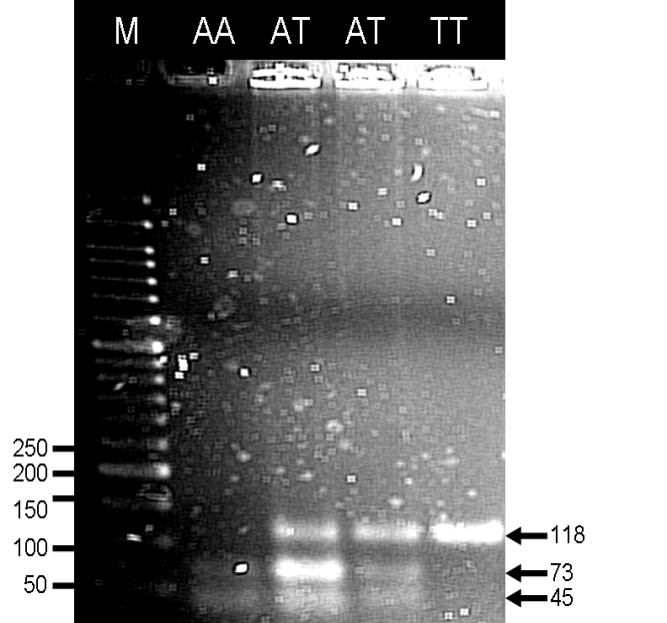
- Five millilitres of blood sample was collected and genomic DNA was then extracted from the nucleated leukocytes using the Wizard® Genomic DNA Purification Kit (Promega Inc., Madison, WI) as mentioned in our previous study[17]. Each of the PCR reaction vial contained 20 µl of solution, containing forward primer (1 μM), reverse primer (1 μM), Taq buffer with ammonium sulphate (1×), Taq DNA polymerase recombinant (1 U/μl)(Fermentas, Lithuania), dNTP (75 μM) (Axygen Biosciences Inc., CA ), MgCl2 (1.25 mM) and 1 µl of dimethyl sulfoxide (DMSO). The PCR was carried out using Biometra T Personal Thermocycler (Biometra GmbH, Germany), beginning with a hot start cycle at 95°C for 5 min; denaturation at 95°C for 30 sec, annealing at 55°C for 30 sec and extension at 72°C for 30 sec for 25 cycles; and a final extension cycle at 72°C for 5 min. The sequences for forward and reverse primers were 5’-CGG CAC AGG TGC ATC GTC TAC CA-3’ and 5’-GAA GAT GGC CAG GGG ACT TGC CA-3’, respectively[15]. Genotypes were determined by Restriction Fragment Length Polymorphism (RFLP), where restriction enzyme HaeIII (isoschizomere BsuRI) (Fermentas Inc., Lithuania) cut the PCR product (118 bp) and formed restriction fragments with the size of 73 bp and 45 bp. Hence, the NPY2R A172T genotypes were AA (digested, homozygous wild-type), AT (digested, heterozygous) and TT (undigested, homozygous mutant) (Figure 1). The fragments were resolved by 3 % agarose gel electrophoresis at constant 90 V for 45 min before staining with ethidium bromide and viewed under a UV transilluminator. The three genotypes were validated by sending to an outsourced DNA sequencing service.
2.3. Statistical Analysis
- The compiled data was analyzed using Statistical Package for Social Sciences, IBM® SPSS® Statistics Version 20.0 (SPSS Inc., IL). The normality of data was examined using One-Sample Kolmogorov-Smirnov Test whereby p > 0.05 indicates that the particular variable is normally distributed.
3. Results
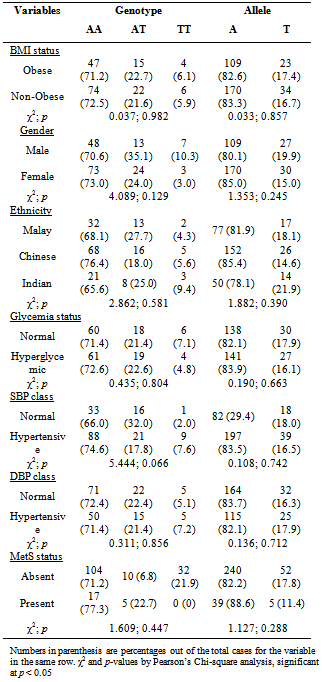
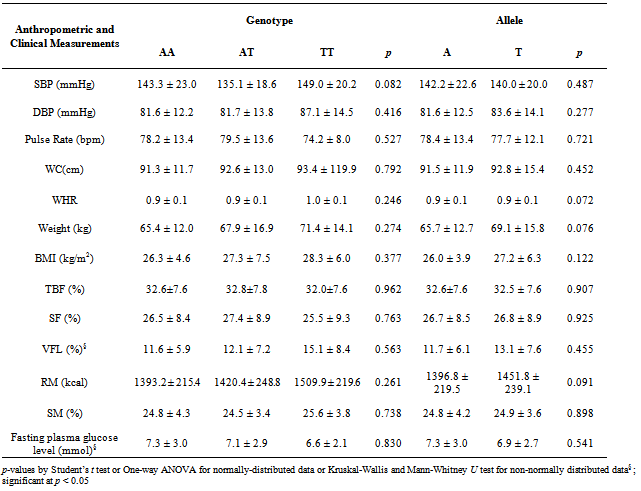
- Based on Table 1, BMI status, gender and ethnicity did not show association with different NPY2R A172T genotypes and alleles. The allele frequency for mutated T allele (or known as Minor Allele Frequency, MAF) was 0.170 and most of the obese subjects carried AA genotype (72.1%) while only 4 had TT genotype. The distribution of genotypes did not deviate significantly from the Hardy-Weinberg Equilibrium. Besides, glycaemia status, hypertensive status (based on SBP or DBP) and MetS status were also all not associated with NPY2R A172T genotypes and alleles (Table 1).With respect to different genotypes and alleles, there were also no significant differences between anthropometric and clinical measurements for different genotypes and alleles (Table 2). Last but not least, out of 68 males, only 3 had MetS while out of 100 females, only 19 had MetS. Therefore, presence of MetS was significantly associated with gender (χ2= 7.569; p = 0.006).
|
4. Discussion
|
5. Conclusions
- NPY2R is a G-protein coupled receptor highly expressed in the ARC of the brain. It exerts anorexigenic or appetite-reducing effect when bound to its ligands. In this study, we screened for the prevalence of a coding missense SNP in the NPY2R gene, namely the A172T variant, to see if the SNP is associated with functional phenotypical effects manifested as obesity and MetS. In conclusion, we found that there was no evidence for an involvement of the NPY2R A172T gene variant in obesity, obesity-related traits and MetS in this multi-ethnic Malaysian study group. The distribution of the genotype and allele frequencies of this gene variant was also not significantly different among gender and ethnic groups. On the basis of the results available so far, the role of NPY2R especially its coding missense substitution A172T gene variant in obesity and MetS remains inconclusive.
ACKNOWLEDGEMENTS
- This project was funded by the Universiti Tunku Abdul Rahman Research Fund (IPSR/RMC/UTARRF/C111/C35). We would like to extend our deepest gratitude to the Kampar District Health Office for granting us permission to carry out this study at the Kampar Health Clinic, the nurses who assisted with the blood sampling, and all the respondents who have volunteered to participate in this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML