Esam A. Gomaa , R. M. Galal
Chemistry Department, Faculty of Science, Mansoura University, Mansoura, 35516, Egypt
Correspondence to: Esam A. Gomaa , Chemistry Department, Faculty of Science, Mansoura University, Mansoura, 35516, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
This paper reports the results on the molar solubility (MS), refractive index (RIN) and molar electrical conductance (EC) of the saturated NaF at different temperatures (293.15, 303.15 and 313.15 K) in mixed ethanol (ETOH) –water solvents. From the experimental part, the molar refraction (RM), limiting molar conductance (LEC), association constants (KASS), free energies of association (GASS) were determined for all solutions by using simple equation from the Shedlovslay model of conductivity.Results predicted the exothermic behaviour of the process in most of the cases. All the measured and calculated parameters for solvation (solubility parameters) and association (conductivity parameters) are increased by increasing the mole fraction of ethanol in the mixtures. This indicates more solute-solvent interaction by more adding ethanol in the mixed solvents.The solubility; conductivity and different thermodynamic data calculated here can easily used for the industial determination of sodium fluoride.
Keywords:
Molar solubility, refractive index, conductance, association constant , free energies of association
1. Introduction
Fluoride in drinking water was originally added to prevent tooth decay. Studies hve now shown that fluoride causes dental decay. Studies have now shown that fluoride causes dental fluorosis in 10% of the population. Research is also linking fluoride to increased risk of cancer (particularly bone cancer), gene mutations , reproductive problems neurotoxicity ( hyper or depressed activity) bone fuorosis (decreasing density) . Fluoride exposure disrupt the synthesis of collagen and leads to breakdown of collagen in bone , muscle. skin, cartilage , lungs , kidney and trachea[1-4] . For industrial uses sodium fluoride is commonly uses in pesticides, including fungicides and insecticides. Various types of adhesives and glues use sodium fluoride as a preservative. Sodium fluoride is also used in making steel and aluminium products. Other industrial uses for sodium fluoride include glass frosting, stainless steel pickling and wood preservation[5].Our purpose is to give new data for solubility and conductivity of sodium fluoride in different ethanol water solvents which can help for the industrial, analytical and biological determination of it.The solubility of an electrolyte is influenced by a wide range of factors, including ion association ,variation in ionicactivity coefficients , complexation and temperature. Solubility is an equilibrium property enable to thermodynamic analysis provided that sufficient information is available[6].Conductivity explains the ion aggregation and the ion solvation and the competitation between them .Two ascpects determine the role of the solvent, its bulk properties and its electron – pair donor and electron – pair acceptor abilities[7].Molar solubility (MS) and electrical conductance (EC) is very important in elucidating not only the behaviour of ions in solution but also in the study of solution structured effects and the preferential solvation of ions by a solvent[8,9].Recently, electrical conductance (EC) studies were done in non-aqueous solvents with the intension of investigation ion-ion and ion-solvent interactions[10,11]. In continuation of this we studied the conducting behaviour of NaF in water, ethanol[EtOH] and their mixtures at different temperatures to elucidate the solvation and association behaviours of NaF under prevailing conditions.
2. Experimental
Sodium fluoride 99%, from Aldrich chemicals Co. Ltd., Gillincham, Dorest – England was used. Absolute ethanol of Al-Gomhoria supplements was used, without further purification.The saturated solutions of sodium fluoride were prepared by dissolving it in H2O-ETOH mixtures in test tubes. The tubes were placed in a shaken thermostat of the typeAssistant for a period of four days, followed by another two days without shaking to reach the necessary equilibrium.The solubility of sodium fluoride were determined gravimetrically, at least three measurements for each solution, by taking 2 ml of each saturated solution and subjecting it to complete evaporation using small aluminium disks heated by I.R Lamp. The conductance of saturated and different concentrations of NaF solutions were measured by using Digital Conductance K120 Conductivity Bridge with cell constant equal 0.991. The refractive indices of the different solutions were measured by using Abbe refractrometer of the type ATAGO 3T, No: 52507, Japan. Weighing four digits Mettler AE 240 balance was used.
3. Results and Discussion
The experimental molar solubility (MS) for NaF in aqueous ethanol solutions were measured in molar scale and tabulated in Tables 1,2,3 at 293.15, 303.15 and 313.15 K. The values of refractive index (RIN) were also measured and tabulated also in these tables at the different temperatures. The salt activity coefficients (log γ±) in each solution were calculated using Debye-Huckel equation[12]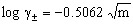 | (1) |
The free energy of solvation GSOL were calculated by using equation (2)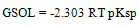 (2)Where pKsp is the solubility product calculated by equation (3)
(2)Where pKsp is the solubility product calculated by equation (3)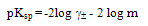 | (3) |
From the experimental refractive indices (RIN), the molar refraction (RM) were calculated[14,15] by applying equation (4),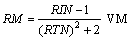 | (4) |
Where VM is the molar volume of NaF in the mixed solvents, calculated by dividing the molecular weight of the salt by the density of solution. VM values ranging from 42.20 cm3/mole to 54.40 cm3/mole for whole the solutions at different temperatures. All the solvation parameters explained before are cited in Tables 1-3 for NaF solutions at different temperatures. The association constant KASS for 1:1 (symmetric) electrolytes can be estimated by applying Fuoss-Sheldovsky equation[16,17]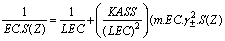 | (5) |
Where EC, molar conductane, LEC, limiting molar conductance and S(Z) is the Fuoss-Sheldorsky factor. This equation is long enough and therefore another simple equation was used as given in equation (6)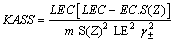 | (6) |
Last equation can be easily applied, knowing that S(Z) factor for NaF and similar salts were found to be approximately one[17].The limiting molar conductance (LEC) was obtained by extrapolating the relation between A and 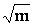 for different solutions to zero concentration.All, the measured and calculated EC , LEC & KASS data are given in Tables 4, 5,& 6 for NaF in the mixed solvents under consideration at different temperatures for different concentrations (m) of the electrolyte. From the association constants KASS the free energy of association GASS were also calculated using equation (7) for NaF solution at different temperatures
for different solutions to zero concentration.All, the measured and calculated EC , LEC & KASS data are given in Tables 4, 5,& 6 for NaF in the mixed solvents under consideration at different temperatures for different concentrations (m) of the electrolyte. From the association constants KASS the free energy of association GASS were also calculated using equation (7) for NaF solution at different temperatures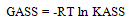 | (7) |
From all the results it was concluded that all the measured and calculated parameters for solvation (solubility parameters) and association (conductivity parameters) are increased by increasing the mole fraction of ethanol in the mixtures[18-22]. This indicates more solute-solvent interaction by more adding ethanol in the mixed solvents.
4. Conclusions
This work introduces a lot of data for NaF which help for its industrial determination. Adding ethanol followed by increase of all the solubility and the thermodynamic parameters, which facilitate the analyst, biochemical analyst and engineering analyst works.| Table 1. Molar solubility (MS), refractive indices (RIN), molar refraction (RM),to activity coefficient (log γ±, solubility product (pKsp) and free energy of solvation (GSOL) for NaF in mixed ethanol solvents at 293.15 K. |
| | Xs of ethanol | MS | RIN | RM | log γ± | pKsp | GSOL (k.J/mole) | | 0 | 1.0238 | 1.33728 | 8.76008 | -0.5121 | -1.0445 | 5.63109 | | 0.0715 | 0.5954 | 1.34373 | 9.17281 | -0.39059 | -1.23148 | 6.9086 | | 0.1703 | 0.2191 | 1.35135 | 9.68601 | -0.2369 | 1.3197 | -7.3979 | | 0.3160 | 0.0555 | 1.35515 | 10.1997 | -0.11925 | 2.2739 | -12.7509 | | 0.550 | 0.0682 | 1.36192 | 11.03206 | -0.13219 | 2.06872 | -11.6015 | | 1.0 | 0.2016 | 1.3618 | 11.79367 | -0.22723 | 0.93654 | -5.2539 |
|
|
| Table 2. Molar solubilities (MS), refractive indices (RIN), molar refraction (RM), log activity coefficient log γ±, solubility product (pKsp) and free energy of solvation (GSOL) for NaF in mixed ethanol-water solvents at 303.15 K. |
| | Xs of ethanol | MS | RIN | RM | log γ± | pKsp | GSOL (k.J/mole) | | 0 | 1.132 | 1.33203 | 8.65719 | -0.5385 | 0.9694 | 5.6145 | | 0.0869 | 0.657 | 1.341055 | 9.14598 | -0.41588 | 0.49046 | 2.8406 | | 0.2024 | 0.321 | 1.34411 | 9.58764 | -0.2867 | -0.4136 | -2.3896 | | 0.3635 | 0.078 | 1.35521 | 10.35736 | -0.14137 | -1.93396 | -11.1958 | | 0.6036 | 0.082 | 1.361181 | 11.13052 | -0.1449 | -1.8825 | -10.903 | | 1.00 | 0.243 | 1.361185 | 11.838009 | -0.24953 | -0.7295 | -4.2259 |
|
|
| Table 3. Molar solubilities (MS), refractive indices (RIN), molar refraction (RM), log activity coefficient log γ±, solubility product (pKsp) and free energy of solvation (GSOL) for NaF in mixed ethanol-water solvents at 313.15 K. |
| | Xs of ethanol | MS | RIN | RM | log γ± | pKsp | GSOL (k.J/mole) | | 0 | 1.2238 | 1.3316 | 8.6566 | -0.5599 | -1.2352 | -7.40255 | | 0.0786 | 0.7604 | 1.3340 | 9.01015 | -0.4414 | -1.12071 | -6.7164 | | 0.1852 | 0.5430 | 1.35212 | 9.86655 | -0.373 | -1.2754 | -7.6494 | | 0.3384 | 0.1021 | 1.3554 | 10.52084 | -0.1617 | -2.3053 | -11.8156 | | 0.577 | 0.091 | 1.036017 | 11.22073 | -0.1527 | -2.3873 | -14.3071 | | 1.0 | 0.3169 | 1.35531 | 11.86548 | -0.2849 | -1.5678 | -9.3938 |
|
|
| Table 4. Molar electrical conductance (EC), limiting molar conductance (LEC), association constants (KASS) and free energies of association (GASS) for different NaF concentrations (M) in mixed aqueous-ethanol solvents at 293.15 K. |
| | Xs mole of fraction | M | EC (m Sem) | LEC (m Sem) | KASS x10-6 | GASS( k.J/mole) | | 0 | 9.61x10-4 | 180 | 903.688 | 49.1656 | -9.495 | | | 1.936x10-3 | 283.33 | | 44.7788 | -9.267 | | | 2.025x10-3 | 443.925 | | 18.8812 | -7.162 | | | 3.969x10-3 | 600.6 | | 3.0567 | -2.723 | | | 4.998x10-3 | 882.35 | | 1.0455 | -0.108 | | | 5.9907x10-3 | 1217.039 | | 0.5412 | +1.496 | | | | | | | | | 0.0715 | 9.61x10-4 | 150 | 716.361 | 96.4945 | -11.138 | | | 1.936x10-3 | 216.66 | | 10.1283 | -5.644 | | | 2.025x10-3 | 280.373 | | 6.9159 | -4.714 | | | 3.969x10-3 | 330.33 | | 2.4369 | -2.171 | | | 4.998x10-3 | 347.82 | | 2.1458 | -1.861 | | | 5.9907x10-3 | 404.411 | | 1.8112 | -1.447 | | | | | | | | | 0.17030 | 9.61x10-4 | 100 | 625.3219 | 53.2847 | 9.692 | | | 1.936x10-3 | 233.33 | | 31.9665 | -8.445 | | | 2.025x10-3 | 280.273 | | 27.4221 | -8.072 | | | 3.969x10-3 | 330.33 | | 9.1013 | -5.383 | | | 4.998x10-3 | 391.304 | | 2.8087 | -2.517 | | | 5.9907x10-3 | 404.411 | | 1.4926 | -0.976 | | | | | | | | | 0.3160 | 9.61x10-4 | 133.33 | 505.659 | 14.9439 | -6.592 | | | 1.936x10-3 | 207.468 | | 8.2681 | -5.149 | | | 2.025x10-3 | 420.42 | | 6.7281 | -4.646 | | | 3.969x10-3 | 588.235 | | 4.1539 | -3.471 | | | 4.998x10-3 | 739.13 | | 0.3606 | -1.273 | | | 5.9907x10-3 | | | | | | | | | | | | | 0.552 | 9.61x10-4 | 80 | 229.274 | 496.1692 | -1.513 | | | 1.936x10-3 | 116.66 | | 38.7865 | -8.917 | | | 2.025x10-3 | 210.28 | | 27.9545 | -8.118 | | | 3.969x10-3 | 330.33 | | 16.992 | -6.820 | | | 4.998x10-3 | 477.94 | | 14.9927 | -6.600 | | | 5.9907x10-3 | 565.217 | | 13.082 | -6.267 | | | | | | | | | 1.0 | 9.61x10-4 | 20 | 206.0889 | 545.0519 | -9.726 | | | 1.936x10-3 | 100 | | 220.0378 | -7.945 | | | 2.025x10-3 | 140.186 | | 26.5983 | -7.705 | | | 3.969x10-3 | 150.15 | | 23.7721 | -7.619 | | | 4.998x10-3 | 173.913 | | 17.8529 | -7.025 | | | 5.9907x10-3 | 183.82 | | 4.4521 | -3.640 |
|
|
| Table 5. Molar electrical conductance (EC),limiting molar conductance (LEC), association constants (KASS) and free energies of association (GASS) for different NaF concentrations (M) in mixed ETOH-H2O solvents at 303.15K. |
| | Xs mole of fraction | M | EC(m Sem) | LEC (m Sem) | KASS x10-6 | GASS k.J | | 0 | 9.61x10-4 | 180 | 1450.037 | 741.008 | -16.657 | | | 1.936x10-3 | 283.33 | | 165.203 | -12.874 | | | 2.025x10-3 | 443.925 | | 119.463 | -12.057 | | | 3.969x10-3 | 600.6 | | 8.8168 | -5.487 | | | 4.998x10-3 | 882.35 | | 8.1709 | -5,269 | | | 5.9907x10-3 | 1217.39 | | 7.570 | -5.104 | | | | | | | | | 0.0809 | 9.61x10-4 | 150 | 675.131 | 255.42 | -13.972 | | | 1.936x10-3 | 216.66 | | 194.806 | -13.289 | | | 2.025x10-3 | 280.373 | | 172.926 | -12.989 | | | 3.969x10-3 | 330.33 | | 165.562 | -12.879 | | | 4.998x10-3 | 347.82 | | 14.228 | -6.693 | | | 5.9907x10-3 | 404.411 | | 10.444 | -5.914 | | | | | | | | | 0.20211 | 9.61x10-4 | 100 | 629.502 | 458.35 | -15.591 | | | 1.936x10-3 | 233.33 | | 274.65 | -14.155 | | | 2.025x10-3 | 280.273 | | 205.35 | -13.422 | | | 3.969x10-3 | 330.33 | | 122.121 | -12.112 | | | 4.998x10-3 | 391.304 | | 76.221 | -10.9214 | | | 5.9907x10-3 | 404.411 | | 50.300 | -9.878 | | | | | | | | | 0.3635 | 9.61x10-4 | 50 | 499.707 | 127.93 | -12.229 | | | 1.936x10-3 | 133.33 | | 46.62 | -9.6855 | | | 2.025x10-3 | 207.468 | | 44.85 | -9.5877 | | | 3.969x10-3 | 420.42 | | 41.991 | -9.4118 | | | 4.998x10-3 | 588.235 | | 3.652 | -3.2656 | | | 5.9907x10-3 | 793.13 | | 3.24 | -2.9673 | | | | | | | | | 0.6036 | 9.61x10-4 | 80 | 411.592 | 248.00 | -13.8985 | | | 1.936x10-3 | 116.66 | | 38.18 | -9.1818 | | | 2.025x10-3 | 210.28 | | 24.30 | -8.0436 | | | 3.969x10-3 | 330.33 | | 7.751 | -5.1630 | | | 4.998x10-3 | 477.94 | | 1.400 | -0.84.85 | | | 5.9907x10-3 | 565.240 | | 1.0691 | -0.1684 | | | | | | | | | 1.00 | 9.61x10-4 | 20 | 369.145 | 92.146 | -11.4027 | | | 1.936x10-3 | 100 | | 37.151 | -9.1128 | | | 2.025x10-3 | 140.186 | | 18.320 | -7.3306 | | | 3.969x10-3 | 150.15 | | 3.647 | -3.2616 | | | 4.998x10-3 | 173.913 | | 2.044 | -3.262 | | | 5.9907x10-3 | 183.82 | | 0.2421 | -1.8021 | | | | | | | +3.5734 |
|
|
| Table 6. Molar electrical conductance (EC), limiting molar conductance(LEC), association constants (KASS) and free energies of association (GASS) for different NaF concentrations (M) in mixed ETOH-H2O solvents at 313.15 K. |
| | Xs mole of fraction | M | EC(m Sem) | LEC (m Sem) | KASS x10-6 | GASS( k.J/mole) | | 0 | 9.61x10-4 | 460 | 2414.716 | 297.56 | -14.826 | | | 1.936x10-3 | 569.948 | | 57.26 | -10.529 | | | 2.025x10-3 | 800 | | 47.8316 | -10.0714 | | | 3.969x10-3 | 1261.682 | | 8.7749 | -5.655 | | | 4.998x10-3 | 1531.153 | | 3.0462 | -2.9005 | | | 5.9907x10-3 | 1695.65 | | 1.2049 | -0.4853 | | | | | | | | | 0.0786 | 9.61x10-4 | 100 | 569.288 | 63.2628 | -10.7987 | | | 1.936x10-3 | 110.294 | | 16.6628 | -7.3255 | | | 2.025x10-3 | 133.33 | | 15.2272 | -7.0900 | | | 3.969x10-3 | 173.913 | | 6.7822 | -4.9848 | | | 4.998x10-3 | 180.294 | | 2.8672 | -2.7609 | | | 5.9907x10-3 | 257.009 | | 2.5966 | -2.4787 | | | | | | | | | 0.1852 | 9.61x10-4 | 110 | 431.279 | 495.867 | -16.1612 | | | 1.936x10-3 | 150 | | 153.6612 | -13.1002 | | | 2.025x10-3 | 210.28 | | 49.9358 | -10.1835 | | | 3.969x10-3 | 280.273 | | 28.2645 | -8.7217 | | | 4.998x10-3 | 330.33 | | 13.7420 | -6.8236 | | | 5.9907x10-3 | 514.705 | | 2.9846 | -2.847 | | | | | | | | | 0.3384 | 9.61x10-4 | 120 | 370.506 | 994.897 | -17.9744 | | | 1.936x10-3 | 166.607 | | 303.059 | -14.8790 | | | 2.025x10-3 | 210.667 | | 66.9891 | -10.9485 | | | 3.969x10-3 | 280.273 | | 35.7653 | -9.3159 | | | 4.998x10-3 | 230.33 | | 8.4775 | -5.5658 | | | 5.9907x10-3 | 514.705 | | 3.3678 | -4.3758 | | | | | | | | | 0.5770 | 9.61x10-4 | 90 | 352.298 | 516.5028 | -16.2674 | | | 1.936x10-3 | 100 | | 255.1022 | -14.4304 | | | 2.025x10-3 | 210.210 | | 24.9736 | -8.3791 | | | 3.969x10-3 | 257.009 | | 17.3687 | -7.4335 | | | 4.998x10-3 | 294.117 | | 7.7073 | -5.3178 | | | 5.9907x10-3 | 347.82 | | 3.5792 | -3.3208 | | | | | | | | | 1.0 | 9.61x10-4 | 60 | 111.6949 | 408.163 | -15.6543 | | | 1.936x10-3 | 83.33 | | 119.399 | -12.4535 | | | 2.025x10-3 | 116.822 | | 38.0233 | -9.4738 | | | 3.969x10-3 | 173.913 | | 7.1179 | -5.1106 | | | 4.998x10-3 | 210.21 | | 7.6428 | -4.9979 | | | 5.9907x10-3 | 220.588 | | 3.1179 | -2.9611 |
|
|
References
| [1] | A.K.Sunsheela and D.Mukerjee,Toxicological European Research,3 (1981) 99. |
| [2] | Y.D.Sharma, Biochemica et Biophysica Acta,715 (1982) 137. |
| [3] | E.Jaouni and D.W. Allman , Journal of Dental Research, 64 (1985) 201. |
| [4] | www.all-natural.com |
| [5] | www.iso.org |
| [6] | Trevor M.Letcher ,” Developments and applications in solubility”. ,The Royal Society of Chemistry, Cambridge, UK.,(2007). |
| [7] | J.M.G.Barthel,H.Krienke and W.Kunz,”Physical Chemistry of Electrolyte Solutions”,Springer-Verlag, Darmstadt , New York , (1998). |
| [8] | B.E. Conway, Ionic hydration in chemistry and biophysics, studies in physical and theoretical Chemistry, Vol. 2, Elsevier Scientific Publishing, Amsterdam, 1981. |
| [9] | Y. Marcus, Ion Solvation, Wiley Inter-Science, New York, 1985. |
| [10] | A.J. Ishwara Bhat, C.B.Susha, Indian J. Chem., 39A (2000) 740. |
| [11] | H. Schneider, in J.F.Coetzee, C.D. Ritchie, Solute-solvent interactions, Marcel Dekker, New York, 1969. |
| [12] | Esam A. Gomaa, Indian Journal of Tech., 24 (1986) 725. |
| [13] | J.I. Kim and E.A. Gomaa, Bull. Soc. Chim. Belg., 90 (1981) 391. |
| [14] | J.B. Hasted, Advances Dielectrics, Chapman and Hall, London (1973). |
| [15] | A.A. El-Khouly, Esam A. Gomaa and S.M. El-Ashry, Bulletin of the Faculty of Science, Mansoura University, 28(2),(2001),137. |
| [16] | T. Shedlovsky and R.L.Kay, J. Phys. Chem., 60, 151 (1956). |
| [17] | E.A. Gomaa, M.A. Hafez and M.N.H. Moussa, Bull. Soc. Chem. Fr. 3, 361 (1986). |
| [18] | K.Rajagopal,S.Edwin Gladson,J.Chem.Thermodynamics,43 (2011)852. |
| [19] | Ewa Kamenska-Piotrowice, Janusz Stangret , JoannaSzymanska-Cybulska,”, Spectrochimica Acta, PartA, 60 (2007) 1. |
| [20] | E.A.Gomaa and B.M.Al-Jahdali,American Journal of Fluid Dynamics, 1(1),(2011)4. |
| [21] | Nagah A. El-Shishtawi,Maany a.Hammada and Esam A. Gomaa, Physical Chemistry, 1(1) ,(2011) 14. |
| [22] | E. A. Gomaa , Analele UniversitatedinBucuresti- Chimie , vol.19,(2010)458. |

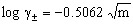
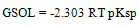 (2)Where pKsp is the solubility product calculated by equation (3)
(2)Where pKsp is the solubility product calculated by equation (3)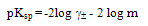
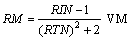
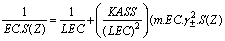
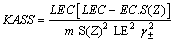
 for different solutions to zero concentration.All, the measured and calculated EC , LEC & KASS data are given in Tables 4, 5,& 6 for NaF in the mixed solvents under consideration at different temperatures for different concentrations (m) of the electrolyte. From the association constants KASS the free energy of association GASS were also calculated using equation (7) for NaF solution at different temperatures
for different solutions to zero concentration.All, the measured and calculated EC , LEC & KASS data are given in Tables 4, 5,& 6 for NaF in the mixed solvents under consideration at different temperatures for different concentrations (m) of the electrolyte. From the association constants KASS the free energy of association GASS were also calculated using equation (7) for NaF solution at different temperatures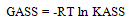
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML