-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2024; 14(3): 45-53
doi:10.5923/j.materials.20241403.01
Received: Dec. 3, 2024; Accepted: Dec. 19, 2024; Published: Dec. 21, 2024

Eco-Friendly Coatings Obtained by Silica from Rice Husk Waste and Hydrophobization with Bio-Based Materials
Liliane C. G.de S. Santos1, Monique A. Cotrim1, Marys L. B. Almeida2, Eliane Ayres1
1Design Postgraduate Program (PPGD), State University of Minas Gerais, Belo Horizonte, Brazil
2Department of Civil Engineering, Federal University of Minas Gerais, Belo Horizonte, Brazil
Correspondence to: Eliane Ayres, Design Postgraduate Program (PPGD), State University of Minas Gerais, Belo Horizonte, Brazil.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study proposes an eco-friendly approach for coating ceramic surfaces to enhance water resistance. Initially, black calcined rice husk was acid-leached and thermally treated to produce light gray ash. From this ash, high-purity silica particles were successfully synthesized using sodium silicate as the precursor. The surface area, determined via BET analysis, was approximately 120 m²/g. Stearic acid and hydrogenated castor oil were evaluated as sustainable hydrophobizers for silica coatings applied to porous ceramic substrates. When hydrogenated castor oil was used, the water contact angle reached 151°. Compared to uncoated substrates, the total color change (ΔE*) was consistently between 5 and 10, which is acceptable for applications not involving the preservation of architectural heritage. This research outlines a cost-effective strategy to convert agro-industrial waste into hydrophobic coatings, offering environmental benefits and contributing to advancements in sustainable construction materials.
Keywords: Agro-industrial waste, Rice husk silica, Bio-based hydrophobizers, Hydrophobic coating
Cite this paper: Liliane C. G.de S. Santos, Monique A. Cotrim, Marys L. B. Almeida, Eliane Ayres, Eco-Friendly Coatings Obtained by Silica from Rice Husk Waste and Hydrophobization with Bio-Based Materials, American Journal of Materials Science, Vol. 14 No. 3, 2024, pp. 45-53. doi: 10.5923/j.materials.20241403.01.
Article Outline
1. Introduction
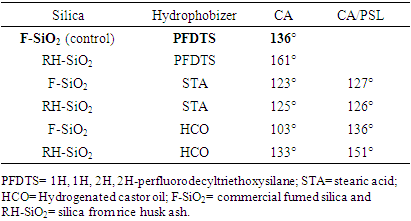
- The upsurge of waste production, driven by population rapid growth, significantly affects both, the environment and economy. Methods for converting these wastes into economically valuable materials can circumvent this trouble (Sarma and Paul, 2024). The population growth puts pressure on the construction industry to build increasingly buildings to meet the needs of current and future generations (Amarasinghe et al., 2024). Consequently, a huge amount of construction and demolition waste, including concrete and bricks, among others, is produced (Amarasinghe et al., 2024). In this regard, green building materials, partially made from wastes, play a crucial role in minimizing the environmental impact of construction (Iwuanyanwu et al., 2024). A large number of possibilities of recycling waste building materials have been reported. Guendouz and Boukhelkhal (2018) replaced cement with different contents of bricks waste powder in mortar composition. Brick waste proved to be suitable as a substitute for cement in the manufacture of common mortars. This diagnosis was due a large extent to improvements in the mechanical performance of the mortars for replacement rates up to 15%. In other studies, recycling floor tile waste and ceramic wastes, such as tile ceramic waste and ceramic sanitary ware, was utilized by partial replacement of natural aggregate in flowable sand concrete (Guendouz et al., 2021; Guendouz and Boukhelkhal, 2019). The use of 60% floor tile waste in this kind of partial replacement led to an increase of 48 and 24% in compressive and flexural strength at 28 days, respectively. Further, the use of 60% of ceramic tile waste or 50% of ceramic sanitary led to an increase of 100 and 51% in compressive strength at 28 days, respectively. The increase in flexural strength at 28 days of age was about 48% with 60% of both ceramic wastes. However, with increasing temperature the compressive strength of the modified concrete decreased, when compared to the reference concrete, after reaching its highest value at 200°C.On the other hand, food agro-industrial by-products such as peels, seeds, stems, bagasse, kernels and husk are rejected as waste and have been considered as environmental pollutants (Gómez-García et al., 2021). Alternatively, the recycling of organic wastes in the field of civil engineering might be considered, as long as the obtained products are not subjected to stringent quality standards (Boukhelkhal et al., 2021). This was the case of the work, which replaced the fine aggregate (sand) in self-compacting mortar by olive core waste with a weight fraction of 10–50% (Boukhelkhal et al., 2021). It was observed a decrease in compressive strength of about 62, 63, 70 and 93% at 7 days for self-compacting mortar with 10, 20, 30, 40 and 50% of olive core waste respectively, compared to control self-compacted mortar. It became 50, 52, 58, 69 and 72% at 28 days with waste contents of 10, 20, 30, 40 and 50%, respectively.According to the authors, the decrease in compressive strength by adding olive core waste is mainly due to the decrease in bulk density of mixtures.Results of other works showed that the use of spent coffee grounds waste, as partial replacement of natural sand in different dosages between 5 and 20%, reduced the mechanical strength of sand concrete mixes (Guendouz and Boukhelkhal, 2018; Guendouz et al., 2022). Nonetheless, the presence of spent coffee grounds caused a reduction in the values of thermal conductivity of the sand concrete (48%). With contents of 15% and 20%, the sand concretes can be regarded as insulating materials (λ<1 W/m °C). As reported by the authors, it is possible to produce thermally insulating eco-friendly concrete for various types of structural elements.Rice husk (RH) is an agricultural waste, either in its raw form or in ash form (RHA), with potential to attain value-added products (Zou and Yang, 2019). When RH is burnt, it gives rise to 17-20% of RHA that is made up of 87-93% of silica (SiO2) and other metallic oxide impurities (Abbas et al., 2024; Endale et al., 2022; Ugheoke and Mamat, 2012). Various metal ions and unburned carbon influence the purity and color of RHA (Chandrasekhar et al., 2005). Particularly, if there is a significant amount of K2O in the husk, during the combustion it can dissociate to form elemental potassium. This causes surface melting, and the carbon gets entrapped in this melt without contact with air, hence hindering its oxidation. This phenomenon contributes to the formation of black particles (Chandrasekhar et al., 2006). For leaching out these impurities, acid treatment with HCl is the most effective (Ugheoke and Mamat, 2012). By controlling the burn of husks after removing metallic oxides, white or grey silica with high purity can be obtained (Chandrasekhar et al., 2006). At higher temperatures, amorphous silica is converted into undesirable crystalline silica (Islam et al., 2024). Crystalline silica has several disadvantages with limited industrial applications (Junaidi et al., 2017; Nzereogu et al., 2023; Carlsen and Saito, 2024). Crystallization can also be accelerated by the presence of K2O in the raw RH (Zou and Yang, 2019). Thus, pre-treating the RH with acid is likewise useful to prevent silica ash crystallization.RHA is considered as the cheapest source for silica gel, which can be extracted by the sol-gel method. In this route, silica is removed from RHA in the form of sodium silicate. The treatment with acid converts the silica into the rigid three-dimensional network of colloidal silica (Lima et al., 2011; Prasad and Pandey, 2012). Silica (SiO2) is widely used as a coating to improve the surface properties of materials such as glass, metal and ceramics (Yuan et al., 2024). As stated in the review by Cannio et al. (2024), antifouling coatings derived from pretreated silica-rich RHA can reduce the cumulative water uptake in the concrete materials. However, first RHA was modified using fluoroalkyl silane in order to enhance the hydrophobicity. In fact, silica is hydrophilic due the presence of silanol groups (Si–OH) on its surface. In order to impart hydrophobicity into the substrate surface, SiO2 particles can be modified through functionalization process (Kumar et al., 2024) or by applying the hydrophobizer solution onto the substrate coated with SiO2 particles (Wu et al., 2022). Li and Xu (2020) obtained silicon dioxide coatings modified by (3-aminopropyl)triethoxysilane (ATS). These materials were obtained by mixing tetraethylorthosilicate, ATS, and OH-terminal polydimethylsiloxane. The effectiveness of the coatings for stone protection was assessed by water uptake and contact angle, among others. Despite the surfaces achieved high repellency, most of formulations are either not environmentally friendly enough to support large-scale manufacturing or prohibitively expensive to scale up (Bayer, 2020). Therefore, low-cost feasible alternatives have been investigated. Nehan et al. (2023) prepared hydrophobic coatings by mixing silica from rice husk ash and polydimethylsiloxane in various ratios. The hydrophobic solutions were coated on glass slides using a brush. They demonstrated the potential use as an anti-water coating on eyeglasses. Castillo and Galarza-Acosta (2024) modified silica nanoparticles, obtained from rice husk, by coating them with stearic acid. For this, they used two different methods: by directly impregnating the nanoparticles with a stearic acid solution, or by dissolving the silica and then mixing it with stearic acid to induce nanoparticle formation in the presence of the acid. In this setting, the present paper aims to present results in the field of sustainable building materials. For this, silica gel derived from rice husk ash (RH-SiO2) has been obtained and characterized. Following this, it was applied as coating onto a porous ceramic substrate. The efficacy of stearic acid and hydrogenated castor oil as hydrophobizers was analysed. Figure 1 presents a graphical diagram of the main stages followed.
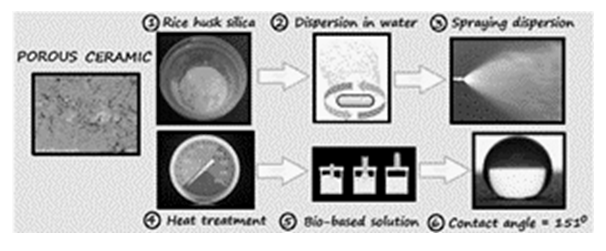 | Figure 1. Graphical diagram with the main stages followed in the present study |
2. Materials and Methods
2.1. Materials
- Carbonized rice husk (CRH) was provided by Indiana Distribution (Embu das Artes, SP, Brazil). 1H,1H,2H,2H-perfluorodecyltriethoxysilane (PFDTES) (code: 658758) and fumed silica (F-SiO2) (code: S5505) were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Hydrogenated castor oil (HCO) was obtained from local commerce (São Paulo, SP, Brazil) and stearic acid (STA) was from Synth (Diadema, SP, Brazil). The bisque ceramic tile (plain, unglazed, once-fired tile), with dimensions 15.3 × 15.3 × 0.3 cm, code SKU AZ0688, from Studio Flama (Belo Horizonte, MG, Brazil) was chosen as substrate. Other common laboratory reagents were used as received, with no purification treatment.
2.2. Production of Silica (RH-SiO2) from CRH
- The following procedure was adapted from other authors (Amutha et al., 2010; Moosa and Saddam, 2017). Firstly, 30g of the CRH was refluxed with 240 mL of HCl (6 M) for 3 h to leach metallic impurities. At the end of reaction, the acid was removed from the CRH by washing with distilled water several times in a vacuum filter using filter paper with a pore size of 0.45 μm. The acid treated CRH was then dried in an oven at 50°C for 6 h. Next, the dried CRH was subjected to thermal treatment at 650°C for 3 h to remove carbonaceous materials and gave rise to rice husk ash (RHA). The following step involved the preparation of sodium silicate. 25 g of treated RHA was stirred with 125 mL of hot water and 22 mL of 3 M sodium hydroxide. The solution was heated at 90°C for 2 h under magnetic stirring and vacuum filtered. The filtrate was the transparent, colorless sodium silicate solution. In the end, the sodium silicate solution was titrated with concentrated sulfuric acid to obtain a gel at pH 8.0. The gel was washed with distilled water until the pH was equal to 7.0 and then dried in an oven at 50°C for 48 h to form white silica powder.
2.3. Coating of RH-SiO2 onto Porous Ceramic Substrate
- Biscuit ceramic tile was used as a porous substrate to apply the coating. The biscuit ceramic tile (or bisque ceramic tile) refers to the tile that underwent the first firing, but it was not glazed. Firstly, biscuit ceramic tile was cut into small pieces (2 cm x 2 cm). The pieces were abundantly washed with ethanol and dried in an oven at 200°C. Then, 0.3 g of RH-SiO2 was dispersed under ultrasonic agitation in 35 mL of deionized water. The dispersion was sprayed onto the surface of samples for 1 min using an airbrush at 15 cm. After drying at room temperature, the coated samples were thermally treated in an oven at 550°C for 1 h and cooled naturally (Wen et al., 2017). As a control, commercial fumed silica (F-SiO2) was used instead of RH-SiO2. In some samples, a solution of expanded polystyrene (EPS) in limonene (40% w/v) was brushing onto the surface of biscuit tile before spraying the silica dispersion. The polymeric film formed after drying acted as a sacrificial layer, which is removed during the thermal treatment (Lejnieks et al., 2010).
2.4. Surface Hydrophobization
- After the thermal treatment, the coated biscuit tile was dipped for 1 min into the hydrophobizer solution, and then dried naturally in air. The solutions stearic acid (STA)/ethanol (2% w/v) and hydrogenated castor oil (HCO)/ethanol (2% w/v) were tested. 1H,1H,2H,2H-perfluorodecyltriethoxysilane (PFDTES) in hexane (1% w/v) was used as a control hydrophobizer (Wen et al., 2017).
2.5. Characterization
2.5.1. Fourier Transform Infrared (FTIR) Spectroscopy
- The Fourier infrared spectrometer (Nicolet, model iS50 FT- IR- Thermo Scientific) in the range of 4000 cm-1 to 650 cm-1 was used to detect the typical sodium silicate and silica bands. Spectra were obtained with an average of 64 scans and a resolution of 2 cm-1, using the Attenuated Total Reflectance (ATR) technique, with diamond crystal.
2.5.2. Specific Surface Area of RH-SiO2 by BET Method
- Brunauer-Emmett-Teller (BET) specific surface area analysis (Bardestani et al., 2019) was performed using 'Quantachrome, model NovaWin2' equipment (Anton Paar GmbH, USA). The sample was analyzed under a nitrogen atmosphere (adsorption-desorption isotherms at 77 K), using multipoint measurement, with relative pressures (P/P0) ranging from 0.01 to 0.99.
2.5.3. X-Ray Diffraction (XRD)
- XRD pattern of RH-SiO2 was obtained using an X-ray diffractometer (PANalyticalX’Pert, Empyrean, Netherlands) operating at 40 kV/40 mA and equipped with a Cu tube (Cu Kα radiation, = 1.5406 Å) and a graphite crystal monochromator. Scanning was performed at 0.06° s-1 for 2θ = 3–90°. The crystallite average size was estimated using Equation 1, called the Debye–Scherrer diffraction equation (Moosa and Saddam, 2017).
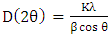 | (1) |
2.5.4. Scanning Electron Microscopy (SEM)
- The surface morphologies were observed with a bench model Hitachi 4000 Plus with an electron beam operating at 5 kV. The images were captured using the backscattered electron detector (BSD).
2.5.5. Contact Angle Measurement (CA)
- The wettability of the coated ceramic substrate was assessed through the values of the contact angle (CA). The measurements were performed by the sessile drop method, with the aid of a DIGIDROP-DI goniometer (GBX Instruments). The results represent the average values of the total (right and left) contact angle with water (10 µL) for individual surface treatment methods. Contact angle values were calculated as mean values of three consecutive recordings, at room temperature, using the Surface Energy mode of the software.
2.5.6. Total Color Change (∆E*)
- The color change (∆E*), considering the uncoated substrate as the reference, was analyzed using a Konica Minolta CM-600D spectrophotometer. The following operating conditions were used: SCE reflectance, scanning from 360 to 740 nm, and observer angle of 10° with D65 illuminant. The data were collected with the Spectra Magic NX software. Every coated sample was measured in three random points, and the color data represented the average of three measurements.
3. Results and Discussion
3.1. Process of Preparing Silica from Rice Husk Ash (RHA) and Characterization
- When rice husk (RH) is calcined, two kinds of materials can produce depending on whether combustion is complete or incomplete. They are rice husk ash (gray or white RHA) and carbonized rice husk (black CRH), respectively (Ugheoke and Mamat, 2012). Herein, black CRH (Figure 2a) was acid treated with HCl, and then to a thermal treatment (650°C) to obtain a light gray ash (RHA) (Figure 2b).
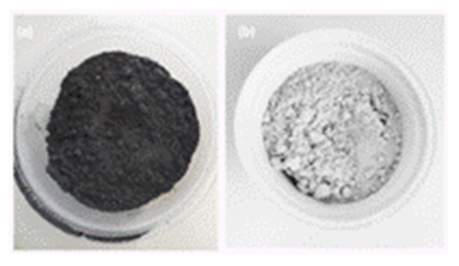 | Figure 2. Photos of CRH as received (a) and RHA after CRH treated with HCl and thermal treatment at 650°C (b) |
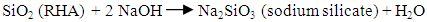 | (2) |
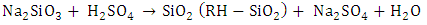 | (3) |
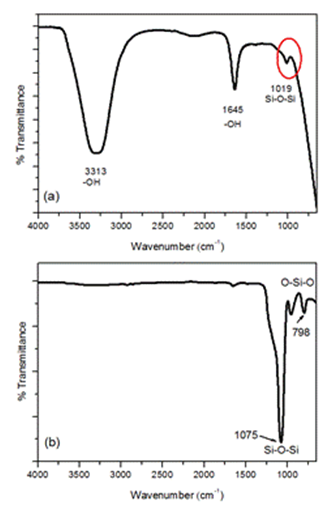 | Figure 3. FTIR spectra of (a) sodium silicate and (b) RH-SiO2 particles |
 | Figure 4. XRD pattern of RH-SiO2. In detail the SEM image of RH-SiO2 |
3.2. Coating and Hydrophobization
- The biscuit ceramic tile although fired, it is not glassy, remains porous, and can absorb water. In some samples, a solution of expanded polystyrene (EPS) in limonene (sacrificial layer) was applied to avoid silica particles penetrate into the substrate pores. Table 1 presents the water contact angle values measured for the different coatings.
|
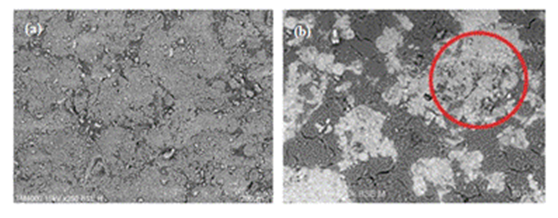 | Figure 5. SEM images of ceramic substrate coated with (a) RH-SiO2 and (b) F-SiO2 |
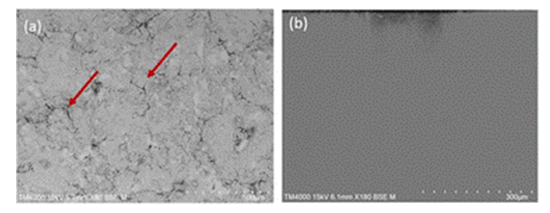 | Figure 6. SEM images of the ceramic substrate: (a) uncoated and (b) coated with polymeric sacrificial layer (PSL) |
 | Figure 7. SEM images of ceramic substrate coated with RH-SiO2 and hydrophobized with STA: (a) without PSL and (b) previously coated with PSL |
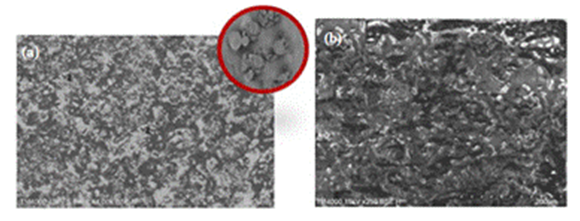 | Figure 8. SEM images of ceramic substrate coated with RH-SiO2 and hydrophobized with HCO: (a) without PSL and (b) previously coated with PSL |
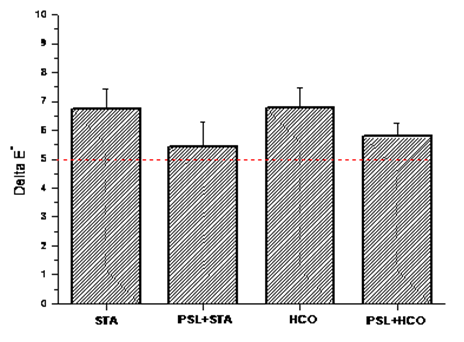
3.3. Optical Appearance of Coated Ceramic Substrate: Total Color Change (∆E*)
- The effect of RH-SiO2 coating on optical appearance of the substrate was performed using the total color change (∆E*) given by Equation 2. The CIELab color space defines a color through three coordinates, L* (brightness: 0= black, 100= white), a*(+ red, - green), and b* (+ yellow, - blue) (Varışli et al., 2023).
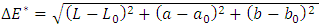 | (4) |
 | Figure 10. Photo of a wall panel made from coated (STA) biscuit ceramic tiles |
4. Conclusions
- This research demonstrated the feasibility of transforming agro-industrial waste into hydrophobic coatings. Silica derived from rice husks was obtained using the sol-gel method, producing high-purity silica from a sodium silicate solution. This silica, dispersed in water, was applied to a porous ceramic substrate, and the coated samples underwent heat treatment. After consolidation of the particles, surface hydrophobization was performed using stearic acid (STA) or hydrogenated castor oil (HCO) as alternatives to fluorinated derivatives.The study highlighted significant results, such as the replacement of environmentally harmful materials with sustainable alternatives. In addition, the use of a polymeric sacrificial layer (PSL) resulted in homogeneous silica coatings and improved contact angles. The roughness conferred by the HCO spherulites further increased water repellency. However, substrates hydrophobized with HCO exhibited higher total color change (ΔE*) values compared to those treated with STA, likely due to the additional surface roughness created by the spherulites.This work highlights the potential of agro-industrial waste to contribute to sustainable construction. The authors hope to inspire continued research and development in waste-derived materials to explore innovative solutions of building materials. Mainly encouraging interdisciplinary collaboration among engineers, chemists, and designers.
ACKNOWLEDGEMENTS
- This work was supported by the Minas Gerais State Research Support Foundation (FAPEMIG), the Coordination for the Improvement of Higher Education Personnel (CAPES) and the National Council for Scientific and Technological Development (CNPq).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML