-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2024; 14(2): 21-30
doi:10.5923/j.materials.20241402.01
Received: Apr. 27, 2024; Accepted: May 20, 2024; Published: May 31, 2024

Structural and Optical Properties of ZnO:Al:Ga Co-Doped Nanoparticles: Investigation on the Effects of pH of the Precursor Solution via a Simple Sol-Gel Route
Kiprotich Bett1, Sharon Kiprotich1, Jatani Ungula2, Warren Andayi1
1Department of Physical and Biological Sciences, Murang’a University of Technology, Murang’a, Kenya
2Department of Applied Sciences, Kenya Methodist University, Meru, Kenya
Correspondence to: Sharon Kiprotich, Department of Physical and Biological Sciences, Murang’a University of Technology, Murang’a, Kenya.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Zinc oxide (ZnO) has unique properties that are suitable for solar cell applications. However, there is need to improve its effectiveness by incorporating dopants into ZnO structure. This study investigates how pH affects the material characteristics of ZnO:Al:Ga co-doped nanoparticles Nps) with the goal of maximizing their application in solar cell technology. After the nanoparticles were synthesized using the sol-gel process at different pH values, their optical, morphological, and structural characteristics were analyzed using various techniques. The observed wurtize crystalline structure of ZnO:Al:Ga Nps was validated by X-ray diffraction (XRD) analysis, with the crystallite size estimated to be ranging from 18.83 to 20.94 nm for pH values 7 to 13. The XRD pattern of the as-prepared ZnO:Al:Ga Nps matched well with the Wurtize ZnO crystal structure with variation in peak intensities observed. Scanning electron microscopy (SEM) displayed aggregation of nanoparticles when the precursor pH increased from 7 to 13. The produced ZnO:Al:Ga Nps showed absorption peaks varying from 380 – 376 nm for precursor pH values 7 – 13, with band gap energy range of 3.26 – 3.29 eV as determined from the UV-Vis analysis. Decreased transmittance was observed with the higher pH values of 11–13 due to its larger particles or aggregation tendencies. In addition, chemical bonds and functional groups were examined using Fourier-transform infrared spectroscopy (FTIR). The results confirmed formation of ZnO:Al:Ga Nps as seen in the FTIR peaks corresponding to Zn-O, Al-O, and Ga-O stretching modes between 400 and 590 cm-1. Additionally, the presence of OH stretching bands above the 3000 cm-1 region indicates surface hydroxylation.
Keywords: Co-doping, ZnO:Al:Ga nanoparticles, Structural properties, Band-gap, FTIR
Cite this paper: Kiprotich Bett, Sharon Kiprotich, Jatani Ungula, Warren Andayi, Structural and Optical Properties of ZnO:Al:Ga Co-Doped Nanoparticles: Investigation on the Effects of pH of the Precursor Solution via a Simple Sol-Gel Route, American Journal of Materials Science, Vol. 14 No. 2, 2024, pp. 21-30. doi: 10.5923/j.materials.20241402.01.
Article Outline
1. Introduction
- Solar energy is leading source of energy for addressing global climate changes due to its reliance on unfriendly energy sources and meeting the world's energy demands for rapidly increasing population growth. In recent years, the search for efficient and sustainable clean energy sources has been intensified as a solution to the negative problems like pollution and the greenhouse effect brought about by the impacts of high dependents on fossil fuels (Huang et al., 2011). The silicon-based solar cells are associated with challenges such as high cost, unreliability, and narrow band gap when it comes to photovoltaic materials. Zinc oxide (ZnO) has attracted a lot of interest due its availability, affordability and unique properties including its ability to be engineered for high efficiency and sustainability of solar energy. The performance of ZnO can be improved by doping with group 13 elements like boron (B) and indium (I) (Bose et al., 2020). However, B and I are not the best dopants because their ionic radii are not as close to Zinc's ionic radius. Aluminum (Al) and Gallium (Ga) are attractive dopants with tailorable properties for high efficiency of ZnO based solar cell applications such as close ionic radii to Zinc, durability, optical transparency, and band-gap tuning (Burunkaya et al., 2010).Previous studies show that synthesis of ZnO at different pH levels have a significant impact on the properties of nanoparticles. It is well known that strongly alkaline solutions improve the optical and structural properties of nanostructures. For example, Sagar et al. (2007) found that the acidic environment inhibits the formation of ZnO nanostructures. According to Sivakumar et al. (2012), there is an improvement in surface morphology as pH levels rise. This was demonstrated by scanning electron microscopy (SEM) images which showed that at lower pH levels, less uniform grain size distribution was observed but at higher pH levels a distinct and enlarged spherical morphology with self-aligned prismatic nanoparticles is obtained. According to Alias et al. (2010), ZnO prepared between pH 8 and pH 11 had good optical properties, with the band gap energy (Eg) ranging from 3.14 to 3.25 eV. The synthesis of the nanomaterial under lower pH 7 - 9 conditions resulted in agglomerated particles. ZnO nanoparticles' suitability for solar cell applications can be greatly impacted by the addition of dopants, which can change the material's energy band gap, transmittance, crystallite size, and light absorption (Chand et al., 2012). Furthermore, the parameters of synthesis, such as the pH of the precursor solution, growth temperatures, aging and calcination are critical in defining the final properties of the nanoparticles. Comprehending the impact of pH on the material properties of ZnO:Al:Ga co-doped nanoparticles is crucial in achieving effective high performance solar cells.The objective of this research is to examine how different pH levels affect the growth of ZnO:Al:Ga nanoparticles for possible solar cell application. The ZnO:Al:Ga co-doped nanoparticles' morphological, structural, and optical properties are all influenced by the synthesis process. The ZnO:Al:Ga co-doped nanoparticles produced by the sol-gel synthesis method were characterized using various techniques to explore the optimum pH of the precursor solution to grow nanoparticles to be incorporated in manufacturing of high performance solar cells.
2. Methodology
- MaterialsZinc acetate, aluminum nitrate, gallium nitrate, sodium hydroxide pellets, Ethanol, deionized water. All the chemicals used were of analytical grade and used as purchased without any alteration.Experimental ProcedureTo study the effects of pH of the precursor solution on the material properties of ZnO:Al:Ga co-doped nanoparticles for solar cell applications, ZnO:Al:Ga co-doped nanoparticles were prepared from Zinc acetate dehydrate, Aluminum nitrate, gallium nitrate, sodium hydroxide, ethanol and deionized water. In the beaker, 2.1951 g of zinc acetate was dissolved in 50 mL of ethanol until clear solution was formed, followed by drop wise addition of 25 mL of 1M sodium hydroxide. The precursor solution was kept under constant stirring speed at growth temperature of 75°C. The pH of the precursor solution was varied for the values: 7, 8, 9, 10, 11, 12 and 13 using 5M Hydrochloric acid and 1M Sodium hydroxide. Thereafter, a solution containing 0.187565 g of aluminum nitrate, 0.12787g of gallium nitrate was added to the precursor solution. After one hour the suspension of ZnO:Al:Ga was removed from hot plate and allowed to age. The ZnO:Al:Ga nanoparticles were then washed with deionized water through decantation. The washed samples were dried at 120°C in the oven and annealed at 600°C. The as-prepared ZnO:Al:Ga co-doped nanoparticles samples were taken for characterization using various techniques: ARL Equinox 100 X-Ray diffractometer (XRD), UV-1800 Shimadzu UV spectrophotometer (UV-Vis), IR spirit Shimadzu Fourier Transform Infrared (FTIR) spectrophotometer, Phenom XLG2 for Scanning Electron Spectroscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS).
3. Results and Discussion
- XRD AnalysisThe XRD analysis of the synthesized ZnO:Al:Ga co-doped nanoparticles is presented in Figure 1, where the diffraction pattern exhibits distinct peaks corresponding to the crystalline phase of hexagonal wurtize present in the sample across all pH values (8, 9, 10, 11, 12, and 13). Wahab et al. (2009) provided similar results, indicating that the diffraction peaks were indexed to the hexagonal wurtzite structure of ZnO that matched with the available Joint Committee on Powder Diffraction Standards for bulk ZnO (JCPDS 36- 1451).
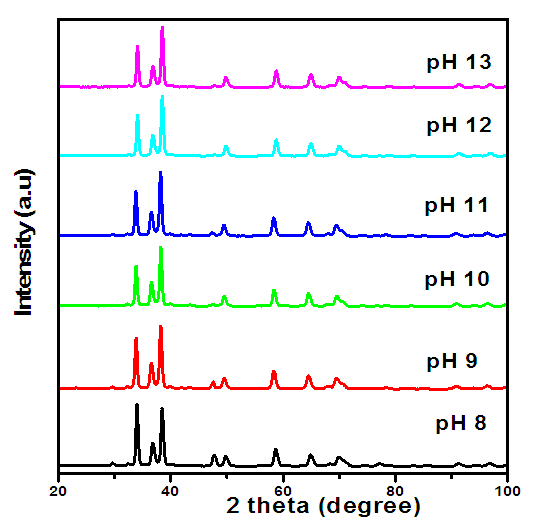 | Figure 1. XRD pattern for ZnO:Al:Ga co-doped nanoparticles synthesized at different pH |
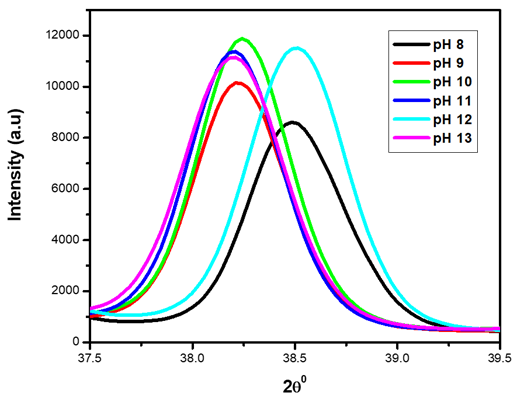 | Figure 2. XRD pattern showing magnified (101) peak for the synthesized ZnO:Al:Ga co-doped nanoparticles under varying pH |
|
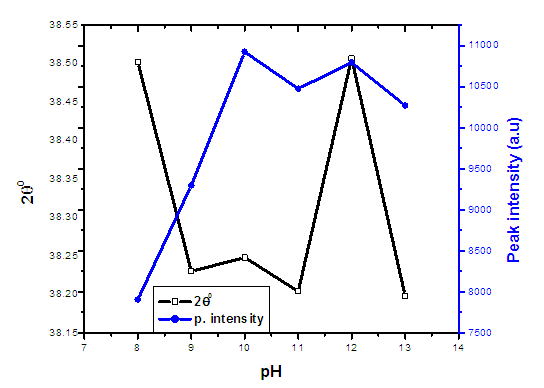 | Figure 3. The graph of 2theta and peak intensity verses pH values of ZnO:Al:Ga co-doped nanoparticles synthesized under varying pH |
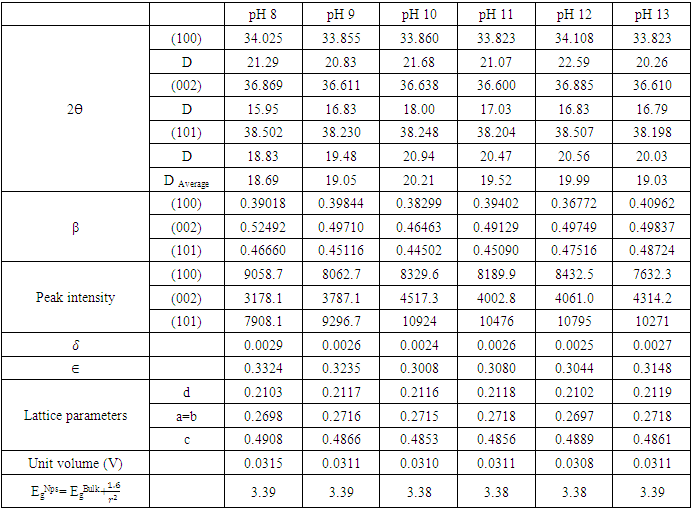
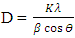 (Gherbi et al., 2022) where D is crystalline size, K is constant (0.89), λ is 0.154056 nm, mean wavelength of CuKα1 radiation, β is full width half maxima (FWHM) and θ is Bragg’s angle in radians. Table 1 shows nano-sized particles with average diameters; 18.69, 19.05, 20.21, 19.52, 19.99 and 19.03 nm for pH values of 8, 9, 10, 12, and 13, respectively. Similar findings were reported by Alias et al. (2010). The sample pH of 8 registered a smallest crystallite size of 18.69 nm while the pH 10 showed the largest crystallite size of 20.21 nm. This is mainly because at pH of 8 the precursor solution is less basic medium whereasat pH of 10, the medium is more alkaline which promotes crystallization (Chen et al., 2020). The size of the ZnO:Al:Ga co-doped crystallites is affected by the size of the FWHM of the XRD peaks. Figure 4 shows the relationship between the crystallite size and the full width at half maximum as pH values of the synthesized ZnO:Al:Ga co-doped nanoparticles are varied. The graph affirmed that crystallite size of nanoparticles are directly proportional to the increase in the pH values on the material properties of the precursor solution while the FWHM decrease with increase in the pH values from 7 – 13. It demonstrates that raising the pH level facilitates the nucleation and development of nanoparticles, the alkaline medium speed up the hydrolysis whereas the acidic medium limits. Alkaline atmosphere promotes hydrolysis when the pH is higher than 7, which increases particle development (Sivakumar et al. (2012) and Jay Chithra et al. (2015).
(Gherbi et al., 2022) where D is crystalline size, K is constant (0.89), λ is 0.154056 nm, mean wavelength of CuKα1 radiation, β is full width half maxima (FWHM) and θ is Bragg’s angle in radians. Table 1 shows nano-sized particles with average diameters; 18.69, 19.05, 20.21, 19.52, 19.99 and 19.03 nm for pH values of 8, 9, 10, 12, and 13, respectively. Similar findings were reported by Alias et al. (2010). The sample pH of 8 registered a smallest crystallite size of 18.69 nm while the pH 10 showed the largest crystallite size of 20.21 nm. This is mainly because at pH of 8 the precursor solution is less basic medium whereasat pH of 10, the medium is more alkaline which promotes crystallization (Chen et al., 2020). The size of the ZnO:Al:Ga co-doped crystallites is affected by the size of the FWHM of the XRD peaks. Figure 4 shows the relationship between the crystallite size and the full width at half maximum as pH values of the synthesized ZnO:Al:Ga co-doped nanoparticles are varied. The graph affirmed that crystallite size of nanoparticles are directly proportional to the increase in the pH values on the material properties of the precursor solution while the FWHM decrease with increase in the pH values from 7 – 13. It demonstrates that raising the pH level facilitates the nucleation and development of nanoparticles, the alkaline medium speed up the hydrolysis whereas the acidic medium limits. Alkaline atmosphere promotes hydrolysis when the pH is higher than 7, which increases particle development (Sivakumar et al. (2012) and Jay Chithra et al. (2015). 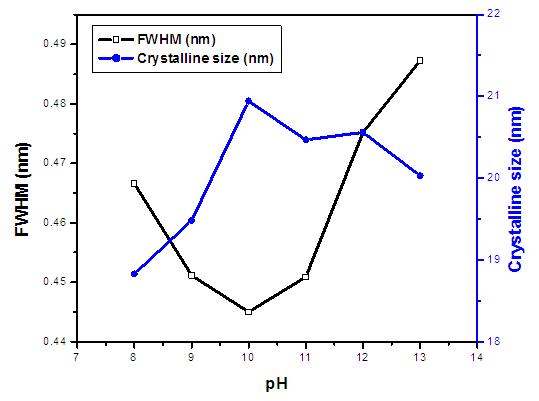 | Figure 4. The variation of FWHM and crystallite size (nm) of prepared ZnO:Al:Ga co-doped nanoparticles at different pH |
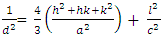 (Jay Chithra et al., 2015 &Verma et al., 2017) where ‘a’ and ‘c’ are the lattice parameters, h, k, and l are the Miller indices and d is the inter-planer spacing. The prepared ZnO:Al:Ga Nps showed ‘a’range from 0.2697 – 0.2718 nm and ‘c’ to be between 0.4853 – 0.4908 nm. The obtained values confirm the presence of imperfections, defects and strain within the nanoparticles since the values deviated slightly from the values of the ZnO bulk structure. To find out the dislocation density of the as-prepared Nps, the formula
(Jay Chithra et al., 2015 &Verma et al., 2017) where ‘a’ and ‘c’ are the lattice parameters, h, k, and l are the Miller indices and d is the inter-planer spacing. The prepared ZnO:Al:Ga Nps showed ‘a’range from 0.2697 – 0.2718 nm and ‘c’ to be between 0.4853 – 0.4908 nm. The obtained values confirm the presence of imperfections, defects and strain within the nanoparticles since the values deviated slightly from the values of the ZnO bulk structure. To find out the dislocation density of the as-prepared Nps, the formula 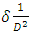 (Kiprotich et al., 2022 & Ungula et al., 2024) where δ is the length of dislocation density and D is the crystalline size was utilized. Also, for the micro-strain
(Kiprotich et al., 2022 & Ungula et al., 2024) where δ is the length of dislocation density and D is the crystalline size was utilized. Also, for the micro-strain 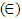 values, the equation
values, the equation 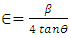 (Kiprotich et al., 2022) where ϴ is the diffraction angle and β is the full width at half maximum (FWHM) were used to find out the cause of the material’s imperfections and defects within the structure. The dislocation density and micro-strain values calculated were recorded in table 1. In figure 5, dislocation density and micro-strain are plotted verses pH values. The graph shows that both the micro-strain and dislocation density of the prepared nanoparticles decrease with increase in pH values up to pH = 10 where it starts to rise. This shows that higher pH of the precursor solution promotes formation of defects in the material.
(Kiprotich et al., 2022) where ϴ is the diffraction angle and β is the full width at half maximum (FWHM) were used to find out the cause of the material’s imperfections and defects within the structure. The dislocation density and micro-strain values calculated were recorded in table 1. In figure 5, dislocation density and micro-strain are plotted verses pH values. The graph shows that both the micro-strain and dislocation density of the prepared nanoparticles decrease with increase in pH values up to pH = 10 where it starts to rise. This shows that higher pH of the precursor solution promotes formation of defects in the material.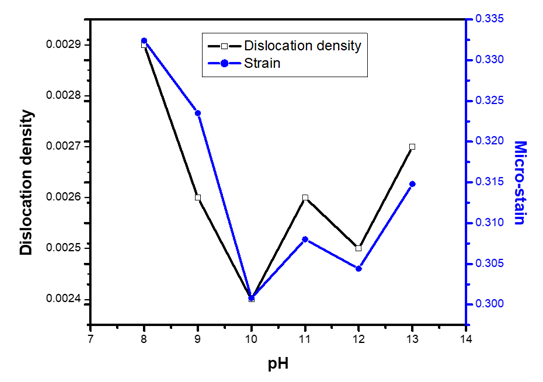 | Figure 5. The graph of dislocation density and micro-strain verses pH values of ZnO:Al:Ga co-doped nanoparticles synthesized under varying pH |
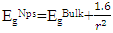 where EgNps is energy band gap of prepared ZnO:Al:Ga co-doped nanoparticles, EgBulk is the energy gap of bulk ZnO (3.37 eV) and r is radius of crystal calculated from XRD data results (Jay Chithra et al. (2015)). From the expression the band-gaps values were obtained and recorded in table 1 as follows 3.39, 3.39, 3.38, 3.38, 3.38 and 3.39 eV for the pH values 8 – 13 respectively. From the estimated band gap values, it can be deduced that the energy gaps of the as prepared nanoparticles is inversely proportional to its crystallite radius. This due to the spatial confinement of electrons within the volumes of nanoparticles increases with decreasing crystallite size, leading to discrete energy levels because of quantum confinement (Ramalingam et al., 2020). The nanoparticles' electrical properties are changed by this confinement, specifically their band gap, which is the energy difference between the lowest unoccupied energy level (conduction band) and the highest occupied energy level (valence band). The energy gap between these levels widens as the crystallite size of the nanoparticle decreases due to quantum confinement.UV-Vis AnalysisOptical properties of the ZnO:Al:Ga co-doped nanoparticles synthesized under varying pH were obtained from the UV-Vis spectroscopy analysis. Figure 6 displays transmittance spectra with comparable patterns at different pH values. The nanoparticles showed higher transmittance a measure of their degree of transparency to incident light at pH 7. This is attributed by reduced agglomeration at pH 7 which is less alkaline that promotes optimal crystallinity which is uniformly minimizing the presence of defects within the material (Liou & Yang. 2011). This enhanced crystallinity reduces light scattering and absorption, allowing more incident light to pass through the nanoparticles without being hindered hence higher transmittance measure.
where EgNps is energy band gap of prepared ZnO:Al:Ga co-doped nanoparticles, EgBulk is the energy gap of bulk ZnO (3.37 eV) and r is radius of crystal calculated from XRD data results (Jay Chithra et al. (2015)). From the expression the band-gaps values were obtained and recorded in table 1 as follows 3.39, 3.39, 3.38, 3.38, 3.38 and 3.39 eV for the pH values 8 – 13 respectively. From the estimated band gap values, it can be deduced that the energy gaps of the as prepared nanoparticles is inversely proportional to its crystallite radius. This due to the spatial confinement of electrons within the volumes of nanoparticles increases with decreasing crystallite size, leading to discrete energy levels because of quantum confinement (Ramalingam et al., 2020). The nanoparticles' electrical properties are changed by this confinement, specifically their band gap, which is the energy difference between the lowest unoccupied energy level (conduction band) and the highest occupied energy level (valence band). The energy gap between these levels widens as the crystallite size of the nanoparticle decreases due to quantum confinement.UV-Vis AnalysisOptical properties of the ZnO:Al:Ga co-doped nanoparticles synthesized under varying pH were obtained from the UV-Vis spectroscopy analysis. Figure 6 displays transmittance spectra with comparable patterns at different pH values. The nanoparticles showed higher transmittance a measure of their degree of transparency to incident light at pH 7. This is attributed by reduced agglomeration at pH 7 which is less alkaline that promotes optimal crystallinity which is uniformly minimizing the presence of defects within the material (Liou & Yang. 2011). This enhanced crystallinity reduces light scattering and absorption, allowing more incident light to pass through the nanoparticles without being hindered hence higher transmittance measure. 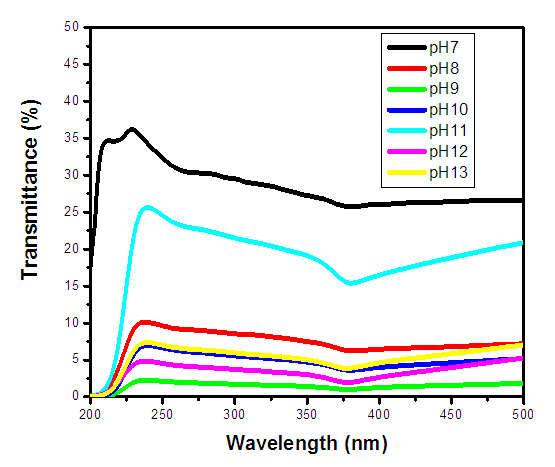 | Figure 6. UV-Vis spectrum showing transmittance (%) against wavelength (nm) of as prepared ZnO:Al:Ga co-doped nanoparticles for varying pH values |
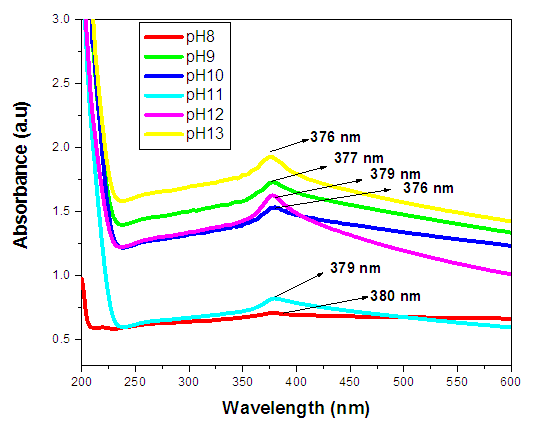 | Figure 7. UV-Vis spectrum absorbance (a.u) against wavelength (nm) of ZnO:Al:Ga co-doped nanoparticles with pH values with respective peaks |
|
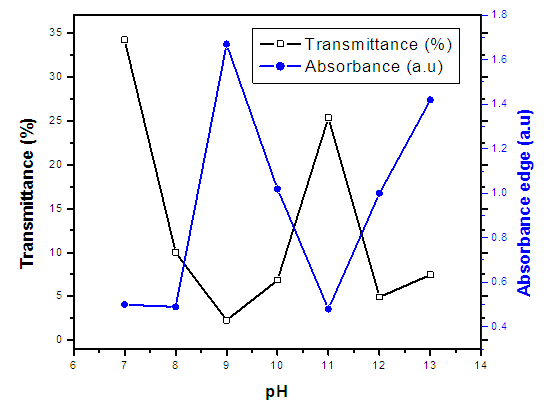 | Figure 8. (%) transmittance and absorption edge verses pH values of synthesized ZnO:Al:Ga co-doped nanoparticles with varying pH values |
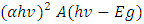 (Ungula et al., 2016) the energy band gap was obtained through extrapolation of the graph of
(Ungula et al., 2016) the energy band gap was obtained through extrapolation of the graph of 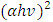 against hv (eV), where A is constant, α is the absorption coefficient (α= 4πk/λ; k is the absorption index), hv is the photon's energy, Eg is the nanoparticles' energy gap. On the other hand this was estimated theoretically using relationship
against hv (eV), where A is constant, α is the absorption coefficient (α= 4πk/λ; k is the absorption index), hv is the photon's energy, Eg is the nanoparticles' energy gap. On the other hand this was estimated theoretically using relationship 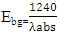 which was obtained by;
which was obtained by;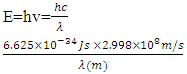 But
But 
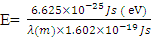 But
But 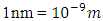
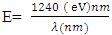 From the mathematical equation of a straight line y=mx+c
From the mathematical equation of a straight line y=mx+c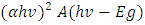 Taking y=0, also will be
Taking y=0, also will be 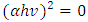
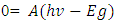 But A=1Now
But A=1Now 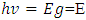
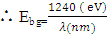 Therefore, the relation
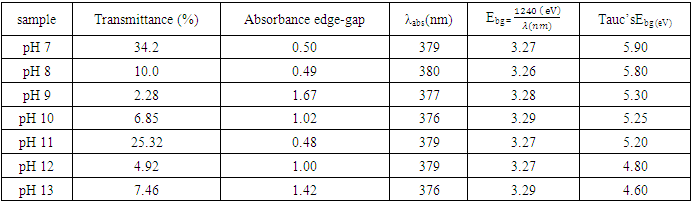
Therefore, the relation 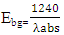 was used to estimate the energy band-gap of the nanoparticles, and the results showed was 3.27, 3.26, 3.28, 3.28, 3.29, 3.27, 3.29, and 3.29 eV for the samples with pH values of 7, 8, 9, 10, 11, 12, and 13, respectively, as summarized in table 2. These estimated results for energy band-gap matches with the values obtained by Jay Chithra et al. (2015), Verma et al. (2017) & Alias et al. (2010). Perhaps, extrapolation of the Tauc’s plot in figure 9 shows different energy band-gaps values that range between 4.6 - 5.8 eV. This is supported by a similar report by Chand et al. (2012) with the result of energy band gap of range 5.57- 5.579 eV. This finding highlighted how crucial pH regulation is for adjusting the optical characteristics of the nanoparticles for particular uses, including solar cell devices, where ideal band gap engineering is necessary for effective light absorption and energy conversion. Since pH regulation is essential for tailoring required optical properties of nanoparticles that are suitable for use in solar cells. By precisely controlling the precursors’ pH synthesis it tune the band gap that enhance light absorption, stability and durability of the material that improved performance and efficiency of solar cells (Ali et al., 2016).
was used to estimate the energy band-gap of the nanoparticles, and the results showed was 3.27, 3.26, 3.28, 3.28, 3.29, 3.27, 3.29, and 3.29 eV for the samples with pH values of 7, 8, 9, 10, 11, 12, and 13, respectively, as summarized in table 2. These estimated results for energy band-gap matches with the values obtained by Jay Chithra et al. (2015), Verma et al. (2017) & Alias et al. (2010). Perhaps, extrapolation of the Tauc’s plot in figure 9 shows different energy band-gaps values that range between 4.6 - 5.8 eV. This is supported by a similar report by Chand et al. (2012) with the result of energy band gap of range 5.57- 5.579 eV. This finding highlighted how crucial pH regulation is for adjusting the optical characteristics of the nanoparticles for particular uses, including solar cell devices, where ideal band gap engineering is necessary for effective light absorption and energy conversion. Since pH regulation is essential for tailoring required optical properties of nanoparticles that are suitable for use in solar cells. By precisely controlling the precursors’ pH synthesis it tune the band gap that enhance light absorption, stability and durability of the material that improved performance and efficiency of solar cells (Ali et al., 2016).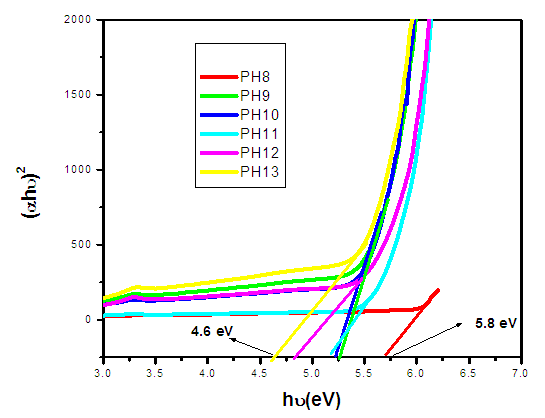 | Figure 9. The Tauc’s plot for determination of energy band-gaps of synthesized ZnO:Al:Ga co-doped nanoparticles with varying pH values |
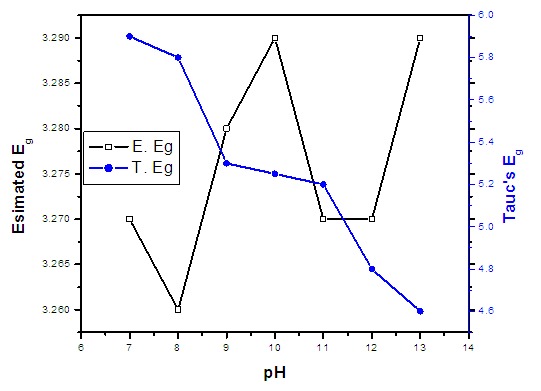 | Figure 10. The graph of estimated energy band gaps of prepared ZnO:Al:Ga co-doped nanoparticles |
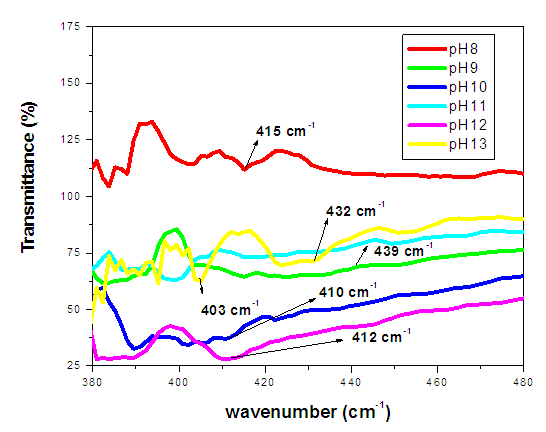 | Figure 11. FTIR spectrum % transmittance against wavenumber (cm-1) of ZnO:Al:Ga co-doped nanoparticles with pH values |
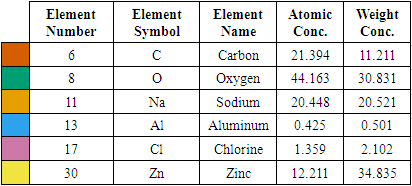
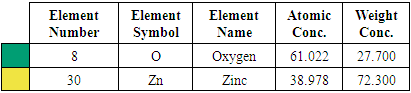
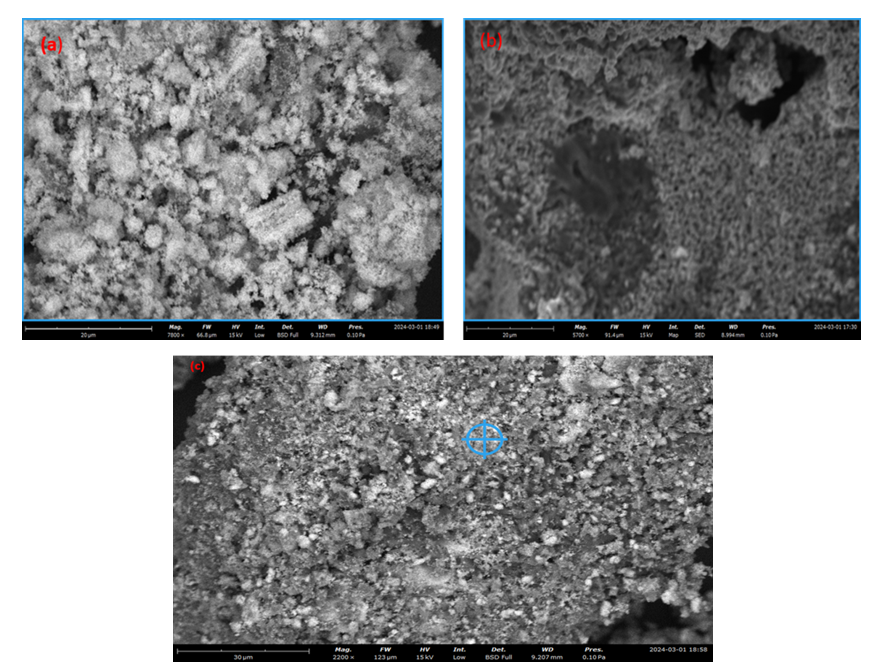 | Figure 12. SEM micrographs of synthesized ZnO:Al:Ga co-doped nanoparticles at (a) pH 8 (b) pH 12 (c) pH 10 |
|
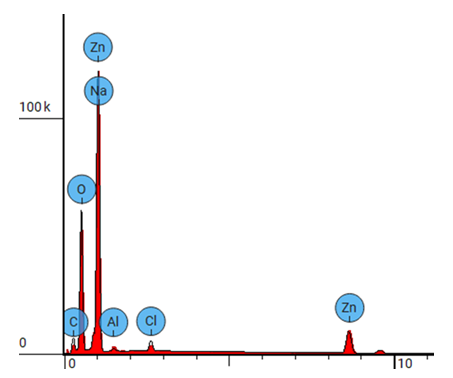 | Figure 13. EDS spectrum for ZnO:Al:Ga Nps prepared at pH 12 |
|
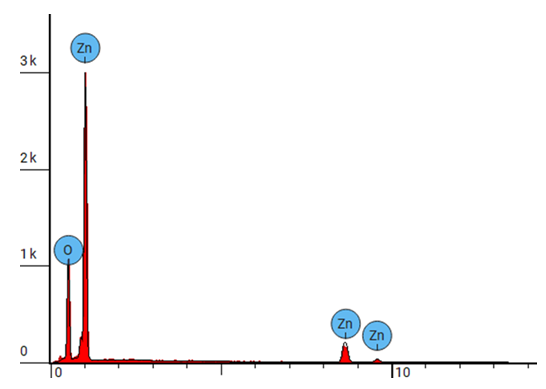 | Figure 14. EDS spectrum for ZnO:Al:Ga NPs sample prepared at pH 8 |
4. Conclusions
- ZnO:Al:Ga co-doped nanoparticles were synthesized and characterized under different pH, which have given important understanding into their potential solar cell applications. It was verified by XRD that the dopants were successfully incorporated into ZnO hexagonal wurtize structure. Changes in the optical properties and band-gap energies of the ZnO:Al:Ga nanoparticles were indicated by changes in transmittance and absorbance, as shown by the UV-Vis spectroscopy investigation. Furthermore, SEM established the morphological and elemental composition of the prepared ZnO:Al:Ga co-doped nanoparticles, while Fourier-transform infrared spectroscopy (FTIR) clarified changes in chemical bonding and functional groups, reflecting the influence of pH on the nanoparticles' structural composition. The findings highlighted pH value of 8 as crucial for synthesizing ZnO:Al:Ga co-doped nanoparticles. Greater transparency and narrower band-gap of 4.8 eV were associated with higher pH values, while lower pH levels generally produced wider band-gaps of 5.8 eV and greater transparency as established by Tauc’s plot contrary to estimated theoretical energy band-gaps between 3.26 – 3.29 eV. The sol-gel synthesis technique used, was successful means of preparing highly crystalline nanoparticles with crystallite size ranging between 18.69 – 20. 94 nm. These results provide important information for enhancing the synthesis procedure and customizing ZnO:Al:Ga co-doped nanoparticle properties for solar cell application.
ACKNOWLEDGEMENTS
- The financial support received from African AI Research grant Award: DSA and Deep Learning Indaba is hereby acknowledged. The authors express gratitude to Murang’a University of Technology for granting access to various synthesis and characterization techniques for the research.
Declaration of Conflict of Interest
- There is no conflict of interest to declare by the authors.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML