| [1] | deDicastillo, C. L., Correa, M. G., Martínez, F. B., Streitt, C., & Galotto, M. J. (2020). Antimicrobial effect of titanium dioxide nanoparticles. Antimicrobial Resistance-A One Health Perspective. |
| [2] | Ilyas, M., Waris, A., Khan, A. U., Zamel, D., Yar, L., Baset, A., & Ahmad, A. (2021). Biological synthesis of titanium dioxide nanoparticles from plants and microorganisms and their potential biomedical applications. Inorganic Chemistry Communications, 133, 108968. |
| [3] | Armaković, S. J., Savanović, M. M., &Armaković, S. (2022). Titanium dioxide as the most used photocatalyst for water purification: An overview. Catalysts, 13(1), 26. |
| [4] | Jaison, A., Mohan, A., & Lee, Y. C. (2023). Recent Developments in Photocatalytic Nanotechnology for Purifying Air Polluted with Volatile Organic Compounds: Effect of Operating Parameters and Catalyst Deactivation. Catalysts, 13(2), 407. |
| [5] | Lavino, S. (2023). Characterization of titanium dioxide coatings for self-cleaning surfaces. |
| [6] | Younas, M., Gondal, M. A., Dastageer, M. A., & Harrabi, K. (2019). Efficient and cost-effective dye-sensitized solar cells using MWCNT-TiO2 nanocomposite as photoanode and MWCNT as Pt-free counter electrode. Solar Energy, 188, 1178-1188. |
| [7] | Padmanabhan, N. T., & John, H. (2020). Titanium dioxide based self-cleaning smart surfaces: A short review. Journal of Environmental Chemical Engineering, 8(5), 104211. |
| [8] | Hiroi, Z. (2022). Inorganic structural chemistry of titanium dioxide polymorphs. Inorganic Chemistry, 61(22), 8393-8401. |
| [9] | Eddy, D. R., Permana, M. D., Sakti, L. K., Sheha, G. A. N., Solihudin, Hidayat, S., ... &Rahayu, I. (2023). Heterophase polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for efficient photocatalyst: fabrication and activity. Nanomaterials, 13(4), 704. |
| [10] | Muthee, D. K., &Dejene, B. F. (2021). Effect of annealing temperature on structural, optical, and photocatalytic properties of titanium dioxide nanoparticles. Heliyon, 7(6), e07269. |
| [11] | Muthee, D. K., &Dejene, B. F. (2020). The effect of tetra isopropyl orthotitanate (TIP) concentration on structural, and luminescence properties of titanium dioxide nanoparticles prepared by sol-gel method. Materials Science in Semiconductor Processing, 106, 104783. |
| [12] | Byrne, C. (2019). Anatase to rutile transition in titanium dioxide photocatalytic nanomaterials (Doctoral dissertation, Institute of Technology, Sligo). |
| [13] | Bakri, A. S., Sahdan, M. Z., Adriyanto, F., Raship, N. A., Said, N. D. M., Abdullah, S. A., & Rahim, M. S. (2017, January). Effect of annealing temperature of titanium dioxide thin films on structural and electrical properties. In AIP conference proceedings (Vol. 1788, No. 1). AIP Publishing. |
| [14] | Borisov, S. V., Magarill, S. A., &Pervukhina, N. V. (2019). Crystallographic Analysis of TiO 2 Polymorphism (Brookite, Anatase, Rutile). journal of structural chemistry, 60, 1783-1789. |
| [15] | Reghunath, S., Pinheiro, D., & KR, S. D. (2021). A review of hierarchical nanostructures of TiO2: Advances and applications. Applied Surface Science Advances, 3, 100063. |
| [16] | Žerjav, G., Žižek, K., Zavašnik, J., & Pintar, A. (2022). Brookite vs. rutile vs. anatase: Whats behind their various photocatalytic activities?. Journal of Environmental Chemical Engineering, 10(3), 107722. |
| [17] | Sajid, M., &Płotka-Wasylka, J. (2020). Nanoparticles: Synthesis, characteristics, and applications in analytical and other sciences. Microchemical Journal, 154, 104623. |
| [18] | A.K.M. Muaz, U. Hashim, M.K.M. Arshad, A.R. Ruslinda, R. Ayub, S.C.B. Gopinath, Effect of Annealing Temperature on Structural, Morphological and Electrical Properties of Nanoparticles TiO2 Thin Films by Sol-Gel Method. Presented at the International Conference on Nano-Electronic Technology Devices and Materials, 2015. |
| [19] | A.S. Bakri, M.Z. Sahdan, F. Adriyanto, N.A. Raship, N.D.M. Said, S.A. Abdullah, M.S. Rahim, Effect of annealing temperature of titanium dioxide thin films on structural and electrical properties, American Institute of Physic. 30030 (2017) 1788–1798. |
| [20] | M. Kumar Singh, M. Singh Mehata, Phase-dependent optical and photocatalytic performance of synthesized titanium dioxide (TiO2) nanoparticles, Optik-International Journal of Light Electronics and Optics. 163011 (2019) 193–204. |
| [21] | Pekov, I. V., Zubkova, N. V., Chukanov, N. V., Yapaskurt, V. O., Turchkova, A. G., Ksenofontov, D. A., &Pushcharovsky, D. Y. (2022). Natisite, Na2TiSiO5, an Indicator Mineral of Hyperagpaitic Hydrothermal Assemblages in the Lovozero and Khibiny Alkaline Plutons, Kola Peninsula: Occurrence, Crystal Chemistry, and Genetic Features. Geology of Ore Deposits, 64(7), 452-469. |
| [22] | Solano Pizarro, R. A., & Herrera Barros, A. P. (2020). Cypermethrin elimination using Fe-TiO2 nanoparticles supported on coconut palm spathe in a solar flat plate photoreactor. Advanced Composites Letters, 29, 2633366X20906164. |
| [23] | RAGU, S., Cho, S., Waqar, A., Ishfaque, A., Choi, D., & Kim, B. Microcrack-Assisted Piezoelectric Acoustic Sensor Based on F-Mwcnts/Batio3@ Pdms Nanocomposite and its Self-Powered Voice Recognition Applications. Available at SSRN 4549162. |
| [24] | Husna, R. A., Suherman, S., & Natsir, T. A. (2023). Effect of Calcination Temperature on the Preparation of Mixed Oxide SnO2/CeO2/TiO2 to Increase the Photodegradation Activity of Methylene Blue under Visible Light. Key Engineering Materials, 949, 75-80. |
| [25] | Saikumari, N., Dev, S. M., &Dev, S. A. (2021). Effect of calcination temperature on the properties and applications of bio extract mediated titanianano particles. Scientific Reports, 11(1), 1734. |
| [26] | Turkten, N., Karatas, Y., Uyguner-Demirel, C. S., &Bekbolet, M. (2023). Preparation of PANI modified TiO2 and characterization under pre-and post-photocatalytic conditions. Environmental Science and Pollution Research, 1-26. |
| [27] | Subasinghe, H. C. S., &Ratnayake, A. S. (2021). Processing of ilmenite into synthetic rutile using ball milling induced sulphurisation and carbothermic reduction. Minerals Engineering, 173, 107197. |
| [28] | Anitha, B., &Khadar, M. A. (2020). Anatase-rutile phase transformation and photocatalysis in peroxide gel route prepared TiO2 nanocrystals: Role of defect states. Solid State Sciences, 108, 106392. |
| [29] | Mustapha, S., Tijani, J. O., Ndamitso, M. M., Abdulkareem, A. S., Shuaib, D. T., Amigun, A. T., &Abubakar, H. L. (2021). Facile synthesis and characterization of TiO2 nanoparticles: X-ray peak profile analysis using Williamson–Hall and Debye–Scherrer methods. International Nano Letters, 11(3), 241-261. |
| [30] | El-Saady, A. A., Farag, A. A. M., Basset, D. M. A., Roushdy, N., & El-Nahass, M. M. (2023). Tailoring the optoelectronic properties of nanocrystalline β-H2Pc films via thermal annealing: Structural, morphological, and optical analyses. Physica B: Condensed Matter, 669, 415339. |
| [31] | Fu, F., Zhang, Y., Zhang, Y., & Chen, Y. (2021). Synthesis of Mn-doped and anatase/rutile mixed-phase TiO 2 nanofibers for high photoactivity performance. Catalysis Science & Technology, 11(12), 4181-4195. |
| [32] | Song, J. J., Deng, J., Zhao, J. Y., Zhang, Y. N., &Shu, C. M. (2021). Comparative analysis of exothermic behaviour of fresh and weathered coal during low-temperature oxidation. Fuel, 289, 119942. |
| [33] | Sivkov, A., Vympina, Y., Ivashutenko, A., Rakhmatullin, I., Shanenkova, Y., Nikitin, D., &Shanenkov, I. (2022). Plasma dynamic synthesis of highly defective fine titanium dioxide with tunable phase composition. Ceramics International, 48(8), 10862-10873. |
| [34] | Yuan, S., Zhu, Y., Huang, M., Liang, S., & Li, Z. (2020). Dislocation-density based crystal plasticity model with hydrogen-enhanced localized plasticity in polycrystalline face-centered cubic metals. Mechanics of Materials, 148, 103472. |
| [35] | Devesa, S., Rooney, A. P., Graça, M. P., Cooper, D., & Costa, L. C. (2021). Williamson-hall analysis in estimation of crystallite size and lattice strain in Bi1. 34Fe0. 66Nb1. 34O6. 35 prepared by the sol-gel method. Materials Science and Engineering: B, 263, 114830. |
| [36] | Zhou, X., Shao, C., Li, X., Wang, X., Guo, X., & Liu, Y. (2018). Three dimensional hierarchical heterostructures of g-C3N4 nanosheets/TiO2 nanofibers: controllable growth via gas-solid reaction and enhanced photocatalytic activity under visible light. Journal of hazardous materials, 344, 113-122. |
| [37] | LandiJr, S., Segundo, I. R., Freitas, E., Vasilevskiy, M., Carneiro, J., & Tavares, C. J. (2022). Use and misuse of the Kubelka-Munk function to obtain the band gap energy from diffuse reflectance measurements. Solid state communications, 341, 114573. |
| [38] | Johannes, A. Z., Pingak, R. K., & Bukit, M. (2020, April). Tauc Plot Software: Calculating energy gap values of organic materials based on Ultraviolet-Visible absorbance spectrum. In IOP conference series: materials science and engineering (Vol. 823, No. 1, p. 012030). IOP Publishing. |
| [39] | Haryński, Ł., Olejnik, A., Grochowska, K., &Siuzdak, K. (2022). A facile method for Tauc exponent and corresponding electronic transitions determination in semiconductors directly from UV–Vis spectroscopy data. Optical Materials, 127, 112205. |
| [40] | Haq, S., Rehman, W., Waseem, M., Meynen, V., Awan, S. U., Khan, A. R., ...& Iqbal, N. (2021). Effect of Annealing Temperature on Structural Phase Transformations and Band Gap Reduction for Photocatalytic Activity of MesoporesTiO 2 Nanocatalysts. Journal of Inorganic and Organometallic Polymers and Materials, 31, 1312-1322. |
| [41] | Žerjav, G., Žižek, K., Zavašnik, J., &Pintar, A. (2022). Brookite vs. rutile vs. anatase: Whats behind their various photocatalytic activities?. Journal of Environmental Chemical Engineering, 10(3), 107722. |
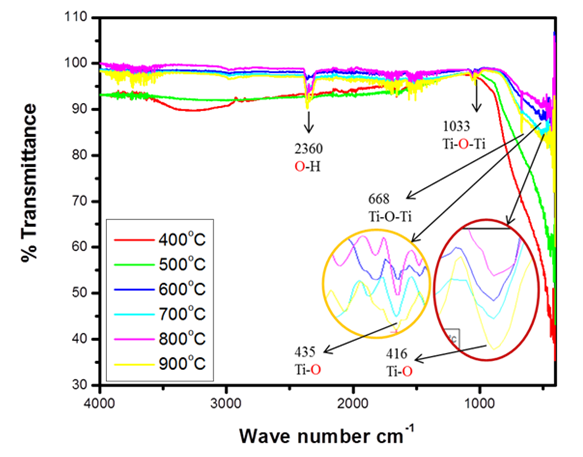
| [42] | Faizan, M., & Ahmad, S. (2021). FTIR and FT-Raman spectra of 6-(dimethylamino) purine and its theoretical studies of anharmonic vibrational analysis using quantum chemical calculations. Vibrational Spectroscopy, 113, 103224. |
| [43] | Ahmad, A., & Ayub, H. (2022). Fourier Transform Infrared Spectroscopy (FTIR) Technique for Food Analysis and Authentication. In Nondestructive Quality Assessment Techniques for Fresh Fruits and Vegetables (pp. 103-142). Singapore: Springer Nature Singapore. |
| [44] | Haghighi, P., & Haghighat, F. (2023). TiO2-based photocatalytic oxidation process for indoor air VOCs removal: A comprehensive review. Building and Environment, 111108. |
| [45] | Zhao, W., Adeel, M., Zhang, P., Zhou, P., Huang, L., Zhao, Y & Rui, Y. (2022). A critical review on surface-modified nano-catalyst application for the photocatalytic degradation of volatile organic compounds. Environmental Science: Nano, 9(1), 61-80. |


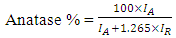
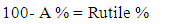
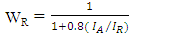
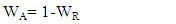

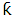 is the shape constant (0.9),
is the shape constant (0.9), 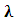 is the X-ray wavelength
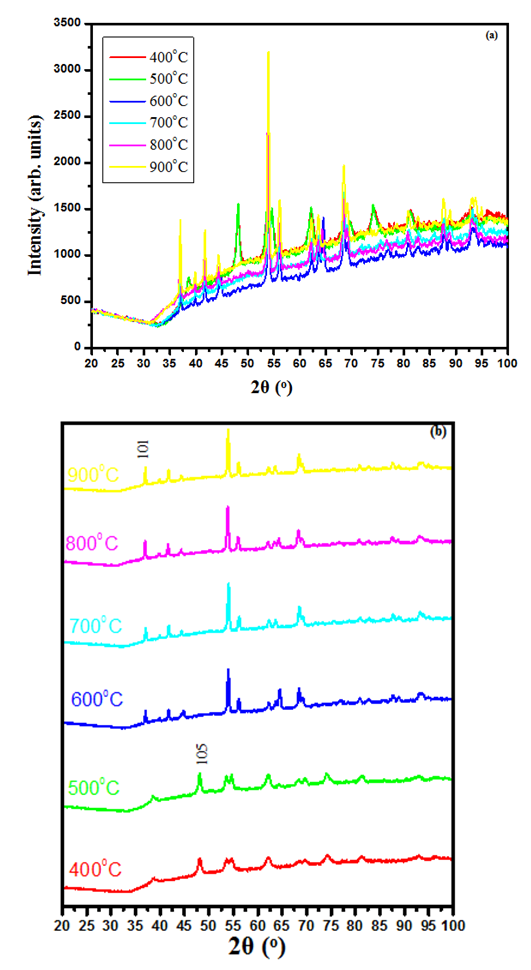
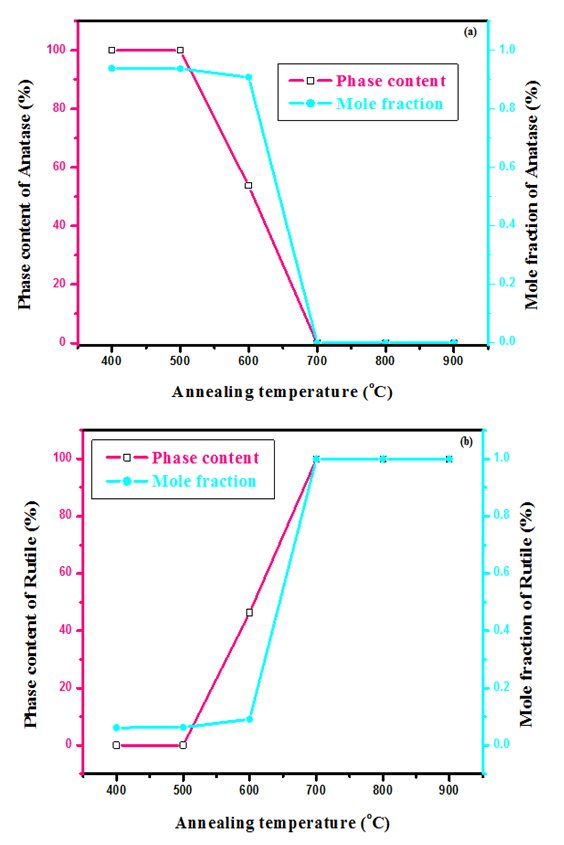
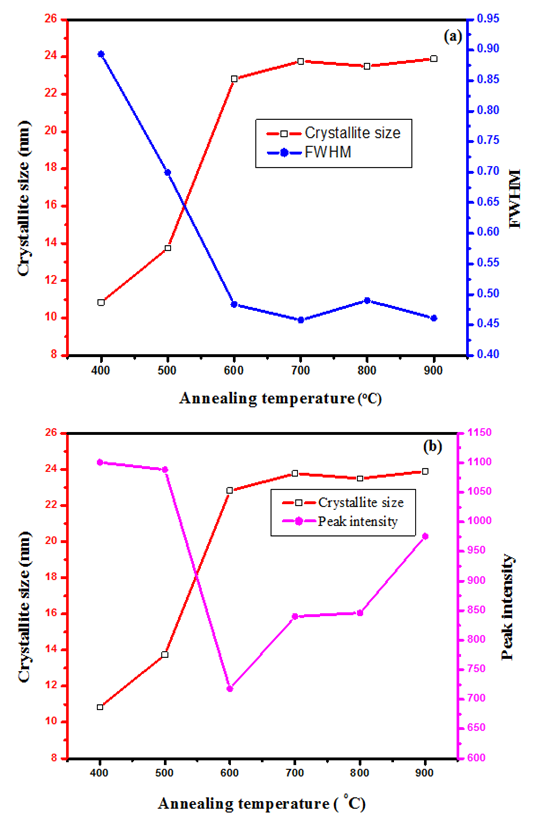
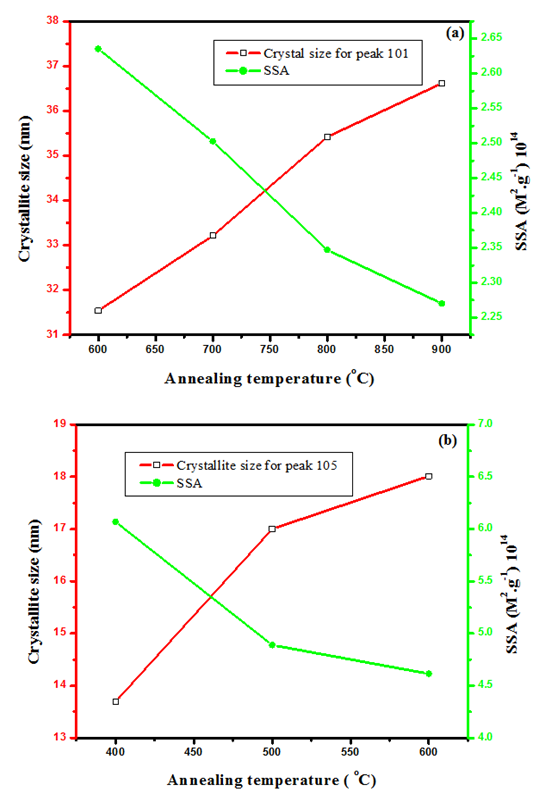
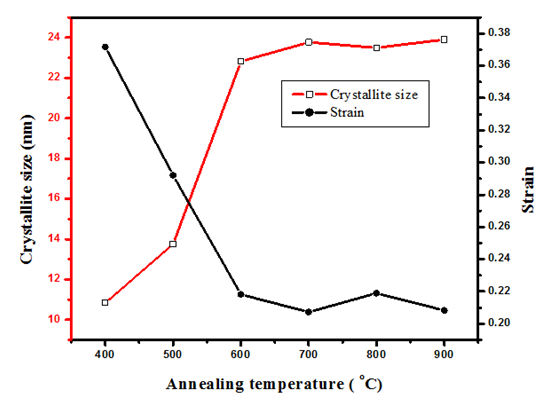
is the X-ray wavelength 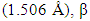 represents the full width at half maximum and θ is the Bragg’s angle in degrees.As depicted in Figure 4(a) average crystallite sizes in (nm) are inversely proportional to their corresponding FWHM values which both appear to be constant at high temperature. Figure 4 (b) shows peak intensity vs crystallite size where the peak intensity in the anatase phase is found to decrease and their crystal size increase as the annealing temperature increases from 400 to 500°C, this is attributed to transformation and phase transition or coexistence with amorphous phase [30]. The peak intensity in the rutile phase increases as the annealing temperature increases due to formation of a more thermodynamic stable phase. This generally indicates improved crystal quality at high temperature; growth temperature rearranges the atoms which in turn increases their particle size [30]. A joint variation was deduced between FWHM, peak intensity and crystallite size where crystallite size is directly proportional to peak intensity and inversely proportional to its corresponding FWHM [10]: Crystal size
represents the full width at half maximum and θ is the Bragg’s angle in degrees.As depicted in Figure 4(a) average crystallite sizes in (nm) are inversely proportional to their corresponding FWHM values which both appear to be constant at high temperature. Figure 4 (b) shows peak intensity vs crystallite size where the peak intensity in the anatase phase is found to decrease and their crystal size increase as the annealing temperature increases from 400 to 500°C, this is attributed to transformation and phase transition or coexistence with amorphous phase [30]. The peak intensity in the rutile phase increases as the annealing temperature increases due to formation of a more thermodynamic stable phase. This generally indicates improved crystal quality at high temperature; growth temperature rearranges the atoms which in turn increases their particle size [30]. A joint variation was deduced between FWHM, peak intensity and crystallite size where crystallite size is directly proportional to peak intensity and inversely proportional to its corresponding FWHM [10]: Crystal size 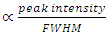 .
.
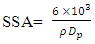
 is the density of TiO2 NPs (4.23g/cm3) and Dp denoted the crystal size obtained by the Debye – scherrer equation [29]. The crystallographic defects or irregularities formed during crystal formation give rise to dislocations. Therefore, dislocation density
is the density of TiO2 NPs (4.23g/cm3) and Dp denoted the crystal size obtained by the Debye – scherrer equation [29]. The crystallographic defects or irregularities formed during crystal formation give rise to dislocations. Therefore, dislocation density  is the length of the dislocation lines per unit volume of the materials crystal calculated using [34] Eq 7
is the length of the dislocation lines per unit volume of the materials crystal calculated using [34] Eq 7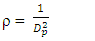
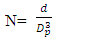
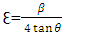
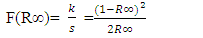
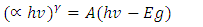
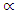 is the linear absorption coefficient, hv is the photon energy, Eg the separation gap between conduction band and valence band and
is the linear absorption coefficient, hv is the photon energy, Eg the separation gap between conduction band and valence band and 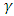 is a constant which depends on the probability of transition; it takes values such as
is a constant which depends on the probability of transition; it takes values such as 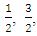 2 and 3 for indirect allowed, indirect forbidden, direct allowed and direct forbidden transitions respectively [39].Replacing
2 and 3 for indirect allowed, indirect forbidden, direct allowed and direct forbidden transitions respectively [39].Replacing 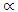 with Kubelka function F(R∞) (Eq 12)
with Kubelka function F(R∞) (Eq 12)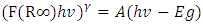
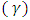 of indirect transition
of indirect transition 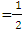 was used as it gave good aquasi. From Eq 12 which corresponds to a linear equation y = mx+c where when extrapolating of the aquasi line to zero absorption coefficient
was used as it gave good aquasi. From Eq 12 which corresponds to a linear equation y = mx+c where when extrapolating of the aquasi line to zero absorption coefficient 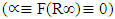 the x intercept gives an estimate value of the band gap(Eg) value. Increased crystal size gives smaller band gap therefore as the annealing temperature increases the crystal size increased resulting in a decreased band gap [40]. The estimated band gap ranges from 3.26 to 3.50 eV; rutile phase has a lower optical band gap as compared to anatase in line with other works [41].
the x intercept gives an estimate value of the band gap(Eg) value. Increased crystal size gives smaller band gap therefore as the annealing temperature increases the crystal size increased resulting in a decreased band gap [40]. The estimated band gap ranges from 3.26 to 3.50 eV; rutile phase has a lower optical band gap as compared to anatase in line with other works [41].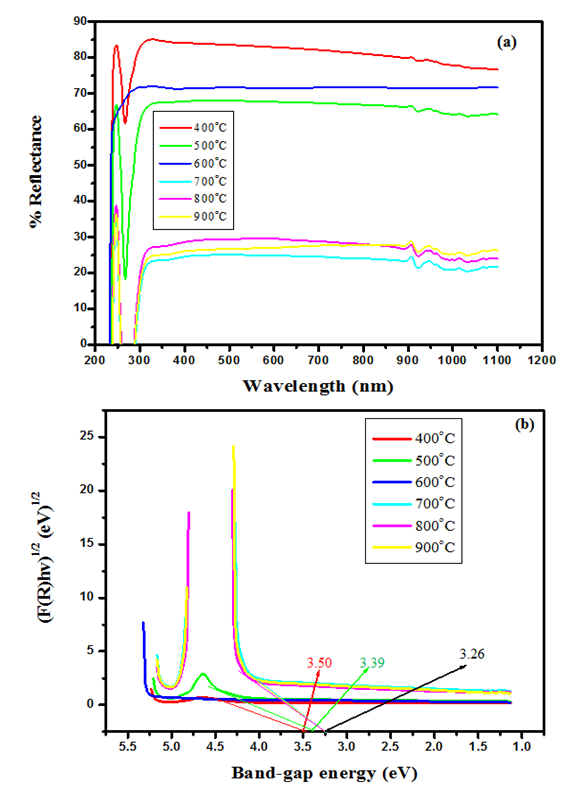
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML