-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2023; 13(1): 1-6
doi:10.5923/j.materials.20231301.01
Received: Jun. 20, 2023; Accepted: Jul. 16, 2023; Published: Jul. 24, 2023

Deposition/Characterization of an Ultrathin Film of Polyallylamine/Polytetrafluoroethylene Colloid Coating on Metallic Substrates
Tarek R. Farhat, Hasan El Rifai, Rana Jisr, Evelyn Patterson, Emily H. Santamaria
Department of Physical Sciences/West Virginia University Institute of Technology, Beckley, WV, USA
Correspondence to: Tarek R. Farhat, Department of Physical Sciences/West Virginia University Institute of Technology, Beckley, WV, USA.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

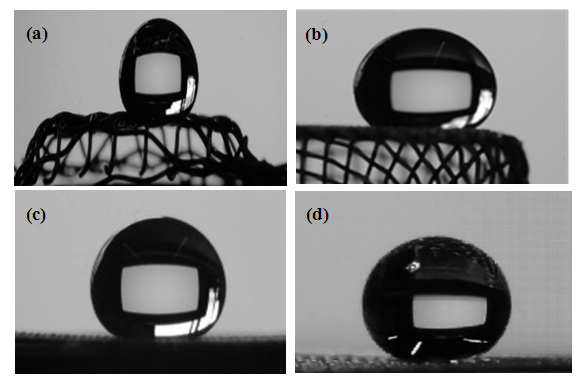
Microparticles of polytetrafluoroethylene (PTFE) were successfully deposited on metal surfaces that are functionalized with a monolayer of polyelectrolyte polyallylamine hydrochloride (PAH). The adherent PTFE colloid created a superhydrophobic ultrathin coating on metal surfaces without using conventional methods of electrostatic/thermal annealing techniques. Deposition of the micron-thin film is at room temperature in aqueous media. The PAH is a diluted polyelectrolyte solution while the PTFE colloid is a 10% colloidal solution. The metallic substrates used are stainless steel plates, SS304 mesh wire, carbon steel, aluminum plates, and aluminum mesh wire. An ultrathin film is deposited starting with PAH to functionalize the metal surface followed by a capping layer of PTFE microparticles to obtain a corrugated surface of low surface energy. The combined effect of the metallic mesh structure, PTFE microparticles, and PTFE low surface energy led to a superhydrophobic coating on the metal surfaces. An instrumental analysis made by infrared spectroscopy, contact angle, and electron microscopy was used to verify the deposition of the ultrathin film coating and the superhydrophobic character. Sessile drops of pure water and solutions exhibited static contact angles up to 160° implying a superhydrophobic character. The PAH/PTFE-coated mesh wires were able to hold a hydrostatic height of 40-50 mm. Our research has applications in the coatings industry that serves corrosion protection, filtration, aqua systems, and others.
Keywords: Superhydrophobic, Ultra hydrophobic, Polyallylamine hydrochloride, Polytetrafluoroethylene, Stainless steel plate, Mesh wire, Static contact angle
Cite this paper: Tarek R. Farhat, Hasan El Rifai, Rana Jisr, Evelyn Patterson, Emily H. Santamaria, Deposition/Characterization of an Ultrathin Film of Polyallylamine/Polytetrafluoroethylene Colloid Coating on Metallic Substrates, American Journal of Materials Science, Vol. 13 No. 1, 2023, pp. 1-6. doi: 10.5923/j.materials.20231301.01.
Article Outline
1. Introduction
- Superhydrophobic films or coatings are an extensive area of research where scientists continue to experiment with a combination of chemicals and techniques to expand applications. In corrosion protection, superhydrophobic coating deprives the process of corrosion from water. For filtration and aqua systems, the repelling characteristics of superhydrophobic films initiate precipitation or vaporization of water. The theory of superhydrophobic surfaces is based on having a low surface energy coating on a rough surface [1,2]. From the many techniques that are used to design superhydrophobic coatings, this paper utilizes the sequential deposition of one bilayer and up to three bilayers used in polyelectrolyte multilayer (PEM) techniques to design ultrathin films. Previous work on the PEM technique used polyelectrolytes (PE) with colloidal particles or hydrophobic PEs to create superhydrophobic coatings [3,4,5,6]. When the polyelectrolyte polyallylamine hydrochloride PAH is used to design hydrophobic coatings, it is usually coupled with an anionic PE (e.g. polystyrene sulfonate PSS, or polyacrylic acid PAA) and silica particles to deposit a PEM film. The film is then capped with a hydrophobic polymer (e.g. perfluorooctyl silane, [7]) to attain superhydrophobic nature. In fact, silica is used extensively in providing the roughness required to design a superhydrophobic coating [8]. The polymer polytetrafluoroethylene PTFE was used as a flexible substrate to support a superhydrophobic coating for biochemical applications, but the PTFE substrate was not part of the hydrophobic ceramic coating [9]. Hydrophobic coating layers with high transmittance were fabricated by optimizing the PTFE colloid content in alumina [10]. Ultra-hydrophobic films were made from titanium dioxide nanoparticles that were fluorinated by the thermal decomposition of polytetrafluoroethylene in a nitrogen atmosphere [11]. Other techniques, roughened the smooth surface of the PTFE substrate by Ar-ion bombardment to design an Ultra-hydrophobic surface [12]. Unlike previous work, our research work succeeded in depositing the PTFE colloid on a metal surface at room temperature under aqueous conditions. Our technique did not use any ceramic powders, thermal annealing, ion-bombardment, and specialty fluorinated molecules to attain a superhydrophobic character. Instead, a metal surface was functionalized by a PAH polyelectrolyte layer followed by one layer of PTFE colloid to design the superhydrophobic coating. Sequential deposition of PAH/PTFE ultrathin film on metallic substrates can be achieved if the deposited film is not left to dry. Up to three bilayers of PAH/PTFE were deposited and characterized. Two types of metallic substrates were used to deposit the PAH/PTFE thin films. The first type of substrates used degreased plates of stainless steel SS304, carbon steel, and aluminum. The second type of substrates used mesh substrates of stainless steel 304 (mesh numbers 16, 38, 70, 120), and aluminum mesh#20. Characterization of the deposited PAH/PTFE thin films on the metallic substrates was made using Fourier Transform infrared FTIR, Contact Angle Measurements CAM, and Scanning Electron Microscopy SEM.
2. Experimental Procedure
- The per-fluorinated coating did not require any chemical synthesis, thus all chemicals involved were purchased. Instruments used in characterization are available in our department.
2.1. Chemicals and Instrumentation
- Polyelectrolyte polyallylamine hydrochloride PAH powder from Alfa Aesar (MW ~ 120K to 200K) and Polytetrafluoroethylene PTFE colloid (60%) from Sigma-Aldrich were used without any further processing. Di-ionized pure water was used to prepare the polyelectrolyte solutions and to dilute the PTFE colloid. The PAH solution used has a concentration of 20 mM dosed with 0.1M NaCl salt. The PTFE colloid suspension was diluted to a 10% colloidal solution with pure water. Instrumental analysis was carried out using a NICOLET iS50 FTIR spectrometer from Thermo-Fisher Scientific, the Contact Angle Measurements (CAM) Goniometer from Ossila Inc., and the PHENOM scanning electron microscope (SEM) analysis from Nanoscience. The metallic mesh substrates from McMaster Carr supplies were of different mesh sizes. The Stainless Steel SS304 has mesh# 120, 70, 38, and 16 while the Aluminum substrate is mesh# 16. The solid plates are SS304 ASTM A240, carbon steel ASTM A216, and aluminum Al-6063. All metallic substrates were rinsed with 95% reagent alcohol, followed by 50/50 peroxide/28% ammonia solution, then plasma etched using a plasma cleaner from Harrick Scientific.In fact, PAH deposition can work with either wet etching or plasma etching depending on the application. The mesh substrates were machined either to fit the end of a glass pipe (GP) of 1.3 cm diameter (Fig-1(a)), analysis, a round spoon (RS) of 1.5 cm diameter and 0.5-1.0 cm depth (Fig-1(b)), and a mini bucket (MB) of 1.2 cm diameter with a 2.0-2.5 cm height, Fig-1(c)).
2.2. Procedure
- A one-bilayer coating of PAH/PTFE, Figure 2, was applied to all plates and mesh substrates as depicted in Figure 2.
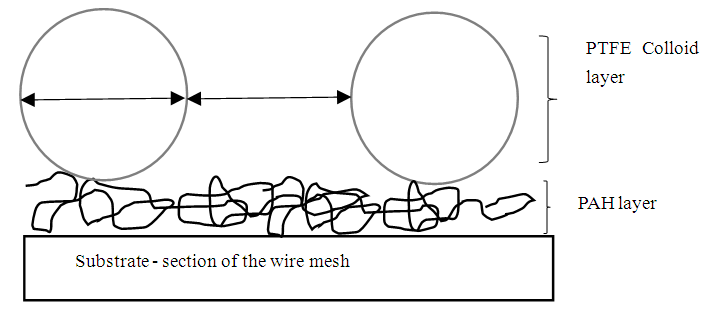 | Figure 2. A schematic showing the deposition of polyallylamine hydrochloride (PAH) on a wire mesh substrate followed by a polytetrafluoroethylene (PTFE) colloidal particles layer to form one bilayer |
3. Results and Discussion
- Surface analysis on the coated and uncoated metallic plates and mesh substrates was made using Fourier Transform Infrared FTIR, Scanning Electron Microscopy SEM, and Contact Angle Measurements CAM.
3.1. FTIR Analysis
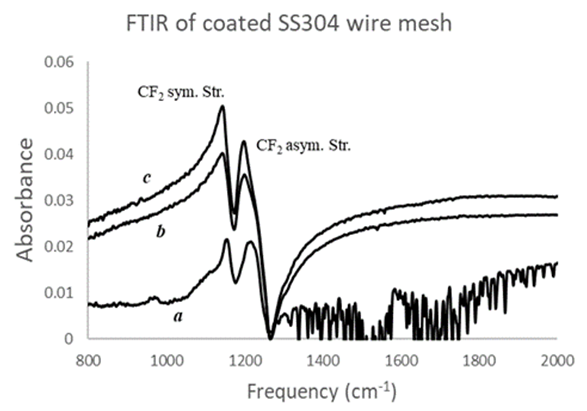
- In Figure 3, the FTIR spectrum of the coated SS304 120 mesh substrate shows the two-strong characteristic peaks of the C-F stretch bands at 1150 cm-1 and 1200 cm-1 that relate to the deposited per-fluorinated PTFE colloid micro-particles [13]. We attempted multiple depositions of two and three bilayers, without curing between layers. Note, at both frequency bands, higher peaks were recorded indicating more deposition of PTFE colloid particles as the number of bilayers increased. The strong trough after the C-F stretch band at 1200 cm-1 is not a derivative peak that can be corrected by the Kramers-Kronig algorithm. It might be a diffractive enhancement of the IR beam going through the coated mesh apertures as more layers get deposited.
3.2. SEM Analysis
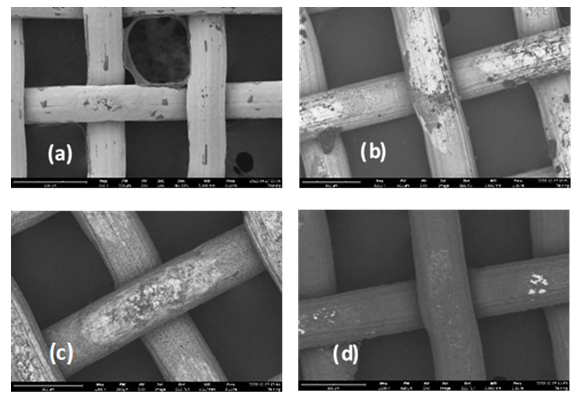
- In Figure 4, the SEM image of the uncoated SS304 wire mesh, Fig-4(a), and the coated wire mesh substrate starting with one bilayer Fig-4(b), two bilayers Fig-4(c), then three bilayers Fig-4(d) are taken at a magnification range from 900X to 1400X. The white grid-like structure represents the uncoated SS304 which is conducting with a high Z value to reflect the BSE, while the dark-grey granulated films are those of the nonconducting low Z PTFE colloid coating allowing greater penetration depth of BSE. Note the intensity of the grey PTFE deposition increases with more bilayers deposited. The three bilayers show that the metal surface is nearly covered by the PAH-PTFE coating. The wire mesh adhered to an aluminum stub using graphite paste tape.
3.3. CAM Analysis
- The wire mesh substrate is a wave-like structure, thus another key structural feature that contributes to the superhydrophobic character is the hydrophobic crests of the wavy wire mesh, Figure 5.
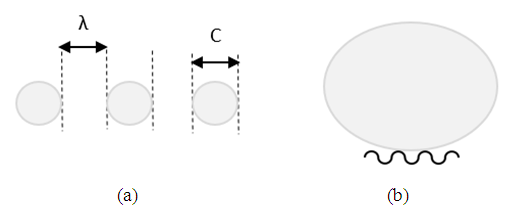 | Figure 5. A simplified schematic of (a) line up of 1 µm size colloidal particles that sets C ~ λ, (b) a liquid drop on a wire mesh |
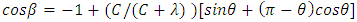 | (1) |
|
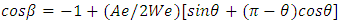 | (2) |
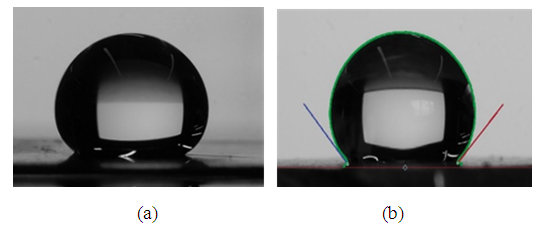 | Figure 6. A sessile drop photos of a water droplet on PAH-PTFE coated metallic plates (a) Stainless steel SS304, (b) Aluminium. Droplet size ~ 20 µL |
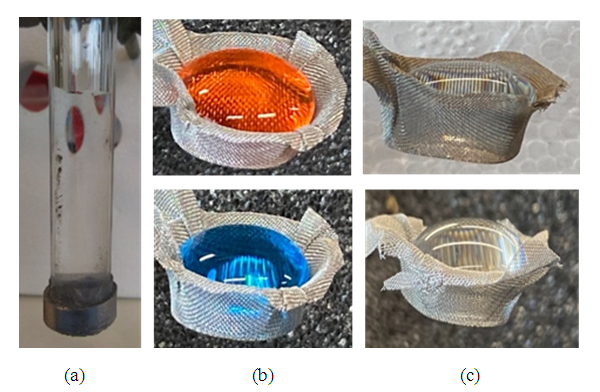 | Figure 8. A PAH/PTFE coated SS304 wire mesh 120 (a) capped GP filled with pure water, (b) MB retaining acidic/basic solutions, (c) MB retaining pure water or salt solutions |
4. Conclusions
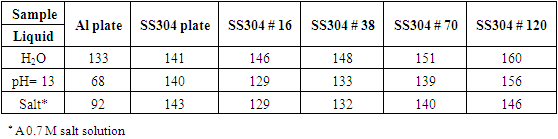
- A bilayer composed of polyelectrolyte polyallylamine hydrochloride (PAH) and a colloidal solution of polytetrafluoroethylene (PTFE) was successfully deposited on stainless steel SS304 and aluminum substrates. The metallic substrates whether a plate form or a mesh wire form (i.e. mesh#16, 38, 70, and 120) were successfully coated with a PAH/PTFE bilayer and multiple bilayers. Surface analysis instrumentation infrared, contact angle, and scanning electron microscopy verified the deposition of the coating on the metallic surfaces used. Static contact angle measurements of sessile drops were made for pure water, acid/base, and salt solutions and ranged from 130° to 160° implying strongly hydrophobic and superhydrophobic character. The wire mesh substrates were superhydrophobic enough to hold water in mini-buckets and glass pipes up to a 5 cm hydrostatic height.
ACKNOWLEDGEMENTS
- The authors would like to thank the West Virginia University Institute of Technology, the Physical Sciences Department, and the NASA West Virginia EPSCoR Consortium for the support of this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML