-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2020; 10(2): 32-41
doi:10.5923/j.materials.20201002.02
Received: Sep. 14, 2020; Accepted: Oct. 26, 2020; Published: Nov. 15, 2020

Complementary Studies on the Electrochemical Behavior of Nano Copper Sulfate with Cefdinir Antibiotic (CFD)
Ahmed S. Abou- Elyazed1, 2, Islam F. Mohamed2, Mohammad Hegazy2
1MIIT Key Laboratory of Critical Materials Technology for New Energy Conversion and Storage, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin, China
2Chemistry Department, Faculty of Science, Menoufia University, Shebin El-Kom, Egypt
Correspondence to: Ahmed S. Abou- Elyazed, MIIT Key Laboratory of Critical Materials Technology for New Energy Conversion and Storage, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin, China.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

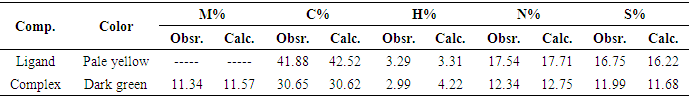
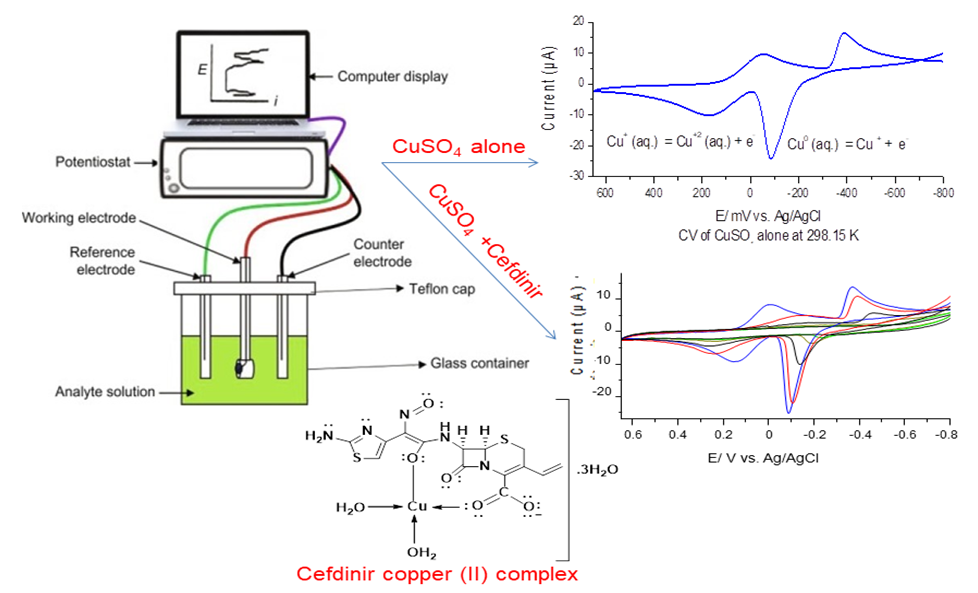
The reaction of nano copper sulfate (NCSu) with Cefdinir antibiotic (CFD) were studied by cyclic voltammetry using a glassy carbon electrode (GCE) in 0.1M KCl as a supporting electrolyte in 5% (v/v) mixed (DMF-H2O) solvent at two temperatures (298.15, 303.15 K). Herein, we observed that copper (II) ion interacted with Cefdinir antibiotic (CFD) to form 1:1 and 1:2 ratio complexes. The stability constants for complex formed and Gibbs free energies of solvation were evaluated. Also the complex formed was characterized by different technique like TEM, FT-IR, TGA and elemental analysis. Furthermore, different thermodynamic parameters; entropies and enthalpies of the system were estimated. The scan rates of copper sulfate (II) in nano size (NCSu) in the presence and absence of Cefdinir antibiotic (CFD) were studied. All mechanism of electrode reactions was discussed and the cefdinir copper (II) complex was synthesized via conventional method and the biological activities of complex was studied.
Keywords: Cyclic voltammetry, Thermodynamics, Cefdinir antibiotic, Stability constant
Cite this paper: Ahmed S. Abou- Elyazed, Islam F. Mohamed, Mohammad Hegazy, Complementary Studies on the Electrochemical Behavior of Nano Copper Sulfate with Cefdinir Antibiotic (CFD), American Journal of Materials Science, Vol. 10 No. 2, 2020, pp. 32-41. doi: 10.5923/j.materials.20201002.02.
Article Outline
1. Introduction
- The unique properties of nanomaterials, salts in nano size, nano fluids and structures on the scale of nanometer have sparked the attention of materials developers. Over the previous decade; materials with structural features (particle size & diameter or grain size, for instance) of at least one dimension in the range 1- 100 nm, have been the concerning of enormous and valuable interest [1-2]. This is a direct result of the high surface (HS) to volume proportion (VP) of nanoparticles compared with macroparticles (MP). Nanomaterials, that branch of materials research, are attracting incredible consideration because of their potential and important applications in different areas such as optoelectronics (OPE) [3], catalysis [4], single-electron transistors and light emitters (LE), [5] nonlinear optical devices (NLD), photoelectrochemical (PE) [6-7] and biological applications. To the best of our knowledge, Copper (II) ions are dissolved in water and play a fundamental role in nutrition to all higher animal and plant life. In animals, including humans, it is presented widely in tissues, liver, muscle and bones. It works as a co-factor in different enzymes and in copper-based pigments [8]. Zampardi, Giorgia, et al. [9] studied the electrochemical behavior of CuO nanoparticles in the assessment of their environmental fate. Also, Liu, Yen-Yu, et al. [10] studied the electrochemical behavior of Cu/poly(2-hydroxy ethyl methacrylate) by cyclic voltammetry. Owing to the accessibility of copper has settled on it a superior decision to work with, in light of the fact that it offers properties like those of other costly respectable metals, including silver and gold. The choice of copper in the present work is ascribed to copper nanoparticles are recorded to have antimicrobial activity against various species of bacteria and fungi [11]. Interestingly, the complexation of medicinal substances with metal ions impacts the bioavailability of drugs in the body. The formation of complex has been recommended as one of the important mechanisms for certain drug action [12]. The metal chelating phenomena are utilized to lessen the poisonous impact of drugs in human physiology [13].Cefdinir drug is a widely used antibiotic throughout the world which is a third generation cephalosporin’s [14]. It is active against both types of bacteria (gram-positive and gram-negative). Bactericidal activity is result of the restraint of cell wall synthesis by acting on penicillin-binding proteins (PBPs) [15]. The structure of Cefdinir (CED) is represented in (Figure 1). Many of reported work describe the complexation reaction between a cephalosporin antibiotic Cefdinir and copper salts. Among of these, Deguchi, Shuhei, et al. [16] suggested structural model for copper (II) Cefdinir complex and described Cefdinir as ligand can form binuclear complex with copper metal ions.
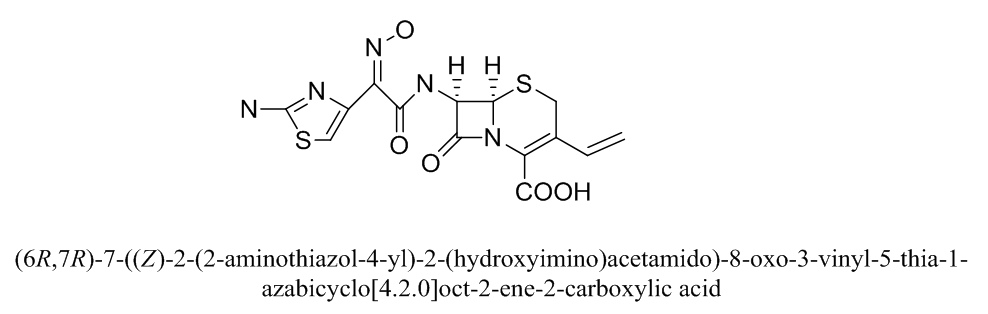 | Figure 1. Structural formula of Cefdinir (CED) antibiotic |
2. Experimental
2.1. Materials
- Cefdinir antibiotic (CED) was purchased from sigma Aldrich and copper sulfate pentahydrate (CuSO4.5H2O) from Merck Germany, dimethylformamide (DMF) from El Nasr pharmaceutical chemicals Co. And used directly without purification, double distilled water was used in this study.
2.2. Preparation of Copper Sulfate in Nano Size (NCSu)
- The copper sulfate in nanosize (NCSu) pentahydrate was prepared as in the literature [17] with some modifications was set up by shaking it in a ball-mill mechanical apparatus assembly of type Retsch MM2000 swing plant for a time of 60 minutes. The mill has a 10 cm3 treated steel tube. Two stain steel balls of 12 mm distance across were utilized. Ball processing was performed at 20225 Hz at room temperature and examined under transmission electron microscopy (TEM).
2.3. Preparation of Cefdinir Copper (II) Complex
- Weight (0.528 g) of Cefdinir drug that dissolved in (15 mL) of 10% mixed solvent (DMF-H2O) and then adding to (0.333 g) of copper sulfate and afterward the mixture was stirred at 150°C for 3h to give the dark green color. The solid obtained was filtered off, washed more times with (ethanol-water) mixture (1:1) and leaving it to dry.
2.4. Characterization of Cefdinir Copper (II) Complex
- The FTIR spectra of the samples were recorded utilizing a Brucker FTIR. The technique includes blending a few amount of a fine powder of the sample with KBr powder in an agate mortar. The mixture was then squeezed by methods of a hydraulic press. The absorbance was consequently enlisted against wavenumber (cm-1). The thermal degradation of Cu (II) complexes was studied using thermo-gravimetric techniques and a temperature range of 27 –800°C.
2.5. Measurements
- The glassy carbon electrode (GCE) was polished to a mirror-like surface with 0.5 and 0.02 µm alumina in doubly distilled water. The experimental solution was deaerated by cleansing for at least 10 minutes with 99.99% pure nitrogen gas. Three electrodes system comprises a glassy carbon electrode (GCE) with surface area (2.83 ×10-3 cm2) as a working electrode, Ag/AgCl (satd. KCl) as reference electrode and platinum wire as the counter electrode was used. Cyclic voltammetry measurement was done by utilizing a Potentiostat Model BASi EPSILON.
3. Results and Discussions
3.1. Characterization of Cefdinir Copper (II) Complex
- The mode of binding of ligand (Cefdinir) to the copper (II) ion was examined by the recording FT-IR spectra of complex as compared with the spectra of free ligand. The spectra of free ligand and the complex are shown in (Figure 2). The FT-IR spectra of Cefdinir drug show a characteristic high intensity band at 1596.77-1559.07 cm-1 which is referred to the ʋ(C=N) vibration. The broad band around 3065.3 cm-1 with two spikes is assigned to H-bonded of NH2 stretching vibration. A strong band at 1767.44-1683.55 and 1623.77 cm-1 in the spectra of cefdinir drug are assigned to ʋ(C=O) of azetidin-2-one moiety, carboxylic group and carbonyl group of secondary amide, respectively. In the complex spectra, it shows that the carbonyl of COOH group exhibited downward shift ʋ(C=O) around 1651.73 cm-1 indicating that the carbonyl of COOH group is co-ordinated to metal ion, while (C=N) group of azomethine moiety are disappeared due to the tautomerization with adjacent carbonyl group of secondary amide that is co-ordinated to metal ion. A broad band around 3749.9-3402.78 cm-1 and two weak bands in the region 873.59-711.6 cm-1 are ascribed to the presence of co-ordinated water due to ʋ(-OH) rocking and wagging modes of vibrations, respectively. The new bands in the region of 586.25-538.04 cm-1 in metal complex assigned to stretching frequency of (M-O). Thus, the FT-IR spectral results provide strong evidences for the complexation of copper sulfate with cefdinir drug. The charge neutrality of the metal complex is maintained by the deprotonating of the COOH group of cefdinir drug prompts the formation of carboxylate ion and by the tautomerization of oxime group with carbonyl group of secondary amide leads to the formation of alkoxide ion. This helps in the formation of ionic bond with the metal. This conflict with the result observed by sharma and coworker [18] and the suggested structure represented in Scheme 1.
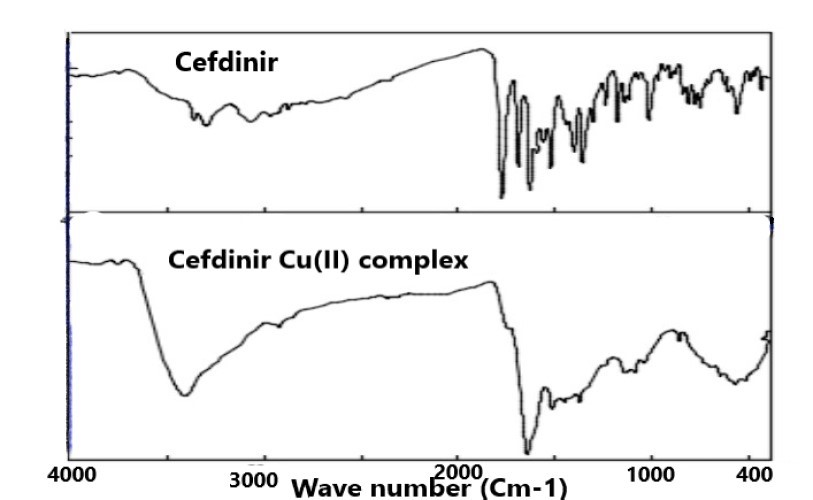 | Figure 2. FT-IR spectra of Cefdinir drug and synthesized compounds |
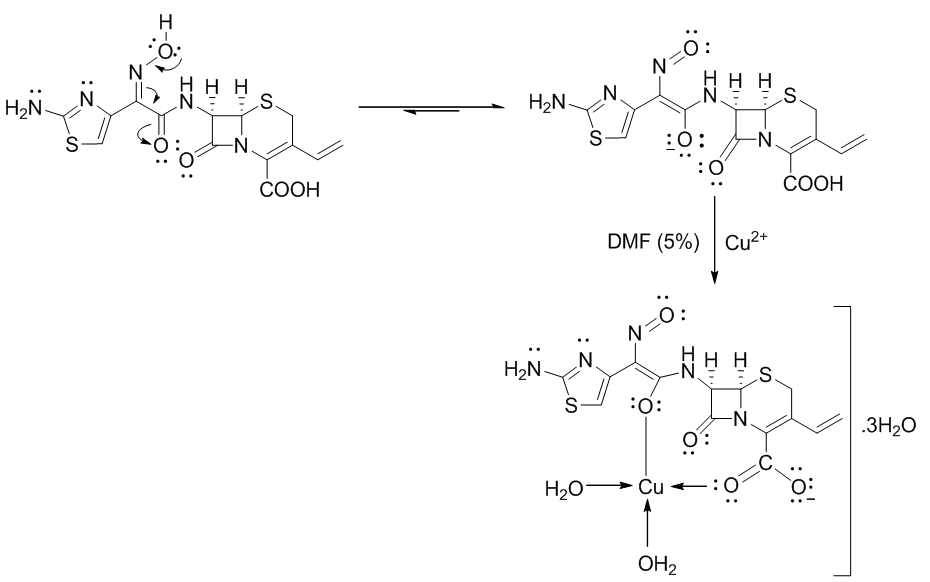 | Scheme 1. Suggested mechanism for the Cefdinir copper (II) complex formation |
|
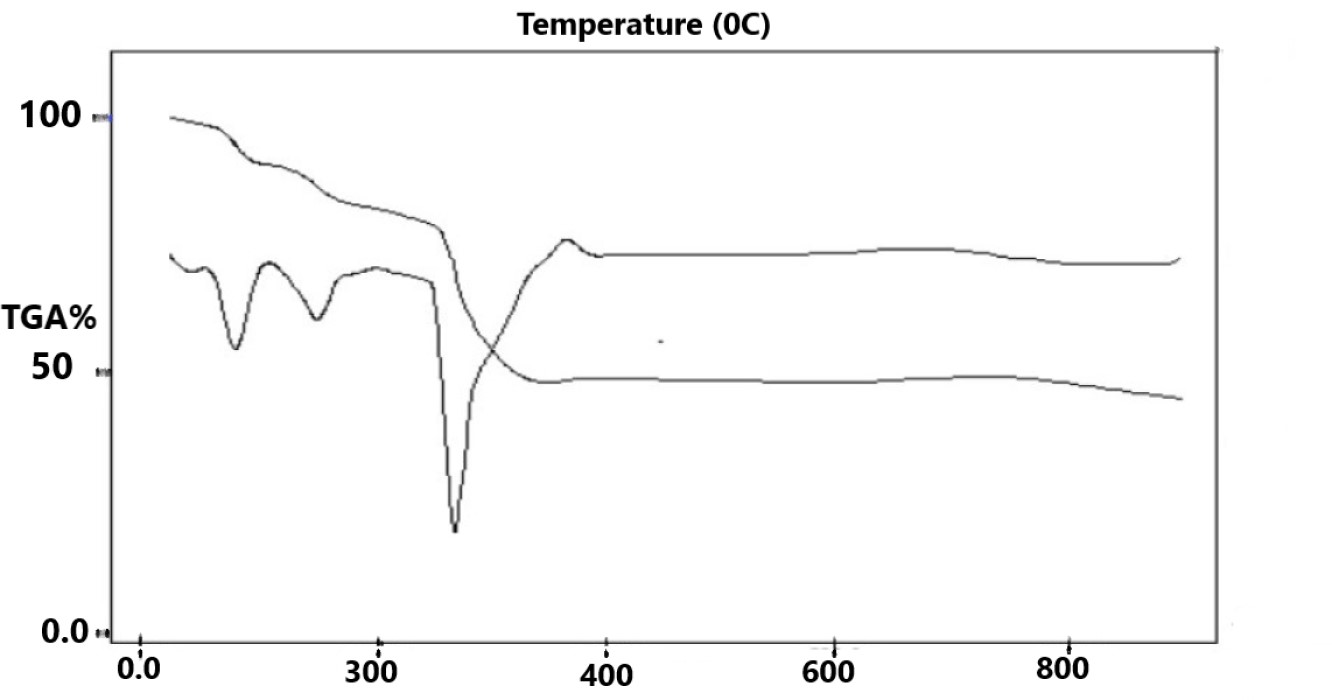 | Figure 3. Thermal gravimetric analysis for Cefdinir copper complex |
3.2. TEM Image for Nano-Copper Sulfate Pentahydrate
- The pictures of nano- CuSO4.5H2O (NCSu) obtained from a transmission electron microscope (TEM) is represented in (Figure 4) from an images we can deduce that CuSO4.5H2O in nano size (NCSu) is either in the form of irregular spheres or deformed spheres, the images show also crystalline form and the dimensions of the particles ranging from 40 to 100 nm. It was concluded from TEM images for ball milled CuSO4 salt presents in nano size and no sharp boundaries between the particles favoring the big solubility of copper sulfate salt in water.
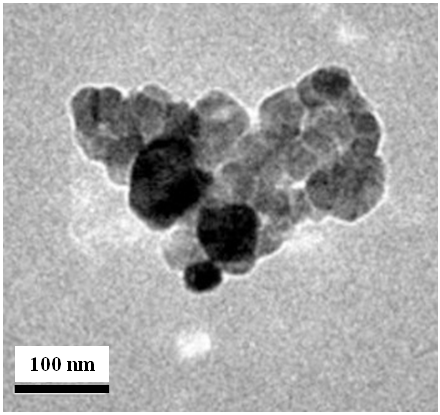 | Figure 4. TEM image of nano- CuSO4.5H2O (NCSu) |
3.3. Electrochemical Behavior of Nano-CuSO4 (NCSu) in the Absence of Ligand (Cefdinir Drug, CED)
- Cyclic voltammogram for both normal and nano size CuSO4 are given for freshly prepared water solutions after ten minutes from preparation indicating about 50% increase in waves height in nano size, therefore our study aim to show the difference in their behaviors. The redox behavior of Cu (II) in nano- CuSO4 (NCSu) was examined in 0.1 M KCl as a supporting electrolyte in 5% mixed solvent (DMF-H2O) by cyclic voltammetry on a glassy carbon electrode (GCE) at different temperatures (298.15, 303.15 K). Small difference in temperature (5 K) was employed as always done in literature to show the behaviors. This process is performed at -800 to 650 mV potential window, current is (100 µA) and 100 mV/S scan rate. In which copper sulfate solution is added in a stepwise way to reach the final concentration is (0.94 mM) as shown in (Figure 5).
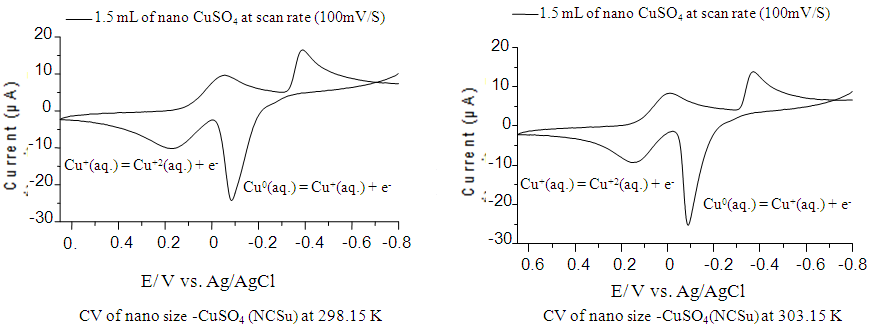 | Figure 5. Cyclic Voltamogram of nano- CuSO4 (NCSU) in 0.1M KCl at 298.15, 303.15 K |
3.4. Electrochemical Behavior of Nano Size - CuSO4 (NCSu) in the Presence of Ligand (Cefdinir Drug) (CFD)
- Herein, we are studying the complexation between cefdinir drug and nano-CuSO4 (NCSu) in 5% (v/v) mixed solvent (DMF-H2O) at different temperatures (298.15, 303.15 K). In which the drug is adding in a stepwise manner. Figure 6 represents the electrochemical behavior of complexation between copper ions and Cefdinir antibiotic (CFD) in 0.1 M KCl in mixed solvent (DMF-H2O) at (298.15, 303.15 K) from -800 mV to 650 mV potential windows, current 100 µA and scan rate 100 mV/S. additionally, the decrease in cathodic and anodic peaks indicate the formation of complex between copper ion and drug. Knowing that Cefdinir antibiotic (CED) shows no waves in this media.
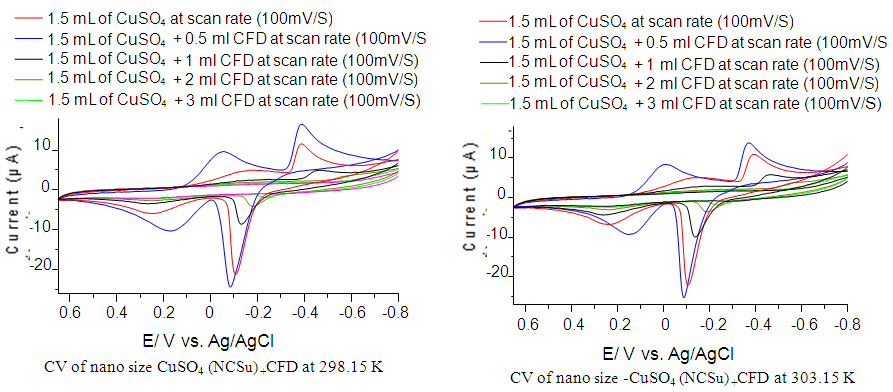 | Figure 6. Cyclic Voltamogram of nano-CuSO4 (NCSU) in the presence of Cefdinir (CED) in 0.1 M KCl at 298.15, 303.15 K |
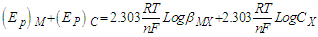 | (1) |
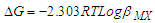 | (2) |
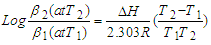 | (3) |
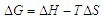 | (4) |
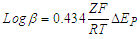 | (5) |
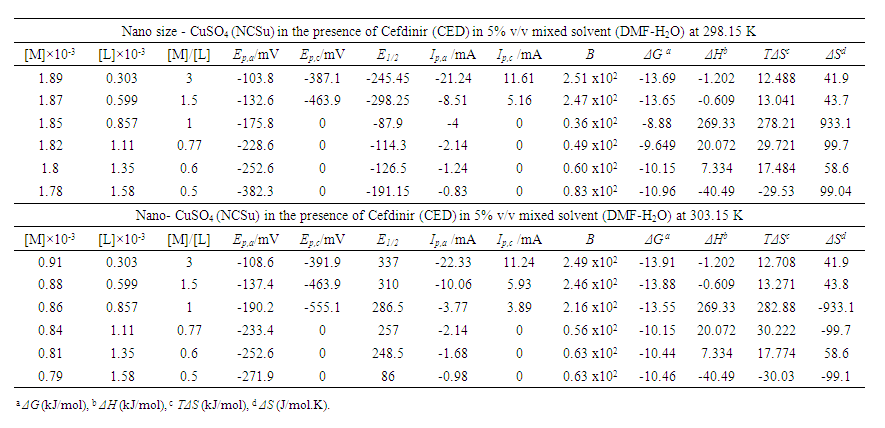 | Table 2. Cyclic voltammetry data of nano size -CuSO4 (NCSu) in the presence of Cefdinir (CED) antibiotic at 298.15, 303.15 K |
3.5. Mechanism of the Redox Reactions
- The copper (II) ions used to show two redox peaks. These two peaks corresponding to the oxidation of copper (0) valent to (I) valent and then the oxidation of copper (I) valent to divalent cupric ions [27-31] The vice versa for the reduction peaks is the reduction of cupric ions to cuprous ion (monovalent), labeled as (A) in (Fig. 5) at 298.15 K then the reduction of monovalent copper to zero valent one labeled as (B) in the same (Fig. 5), copper metal involving two electrons the (C) stripping wave as explained before and (D) are the oxidation peaks explained above, in this media done versus silver/silver chloride electrode as follows:
 | (A) |
 | (B) |
3.6. Variation of the Scan Rate
- Cyclic voltamogram of nano-CuSO4 (NCSu) in absence and presence of Cefdinir antibiotic in 0.1 M KCl at -800 mV to 650 mV potential windows, current 100 µA and different scan rates (50, 100, 150, 200 and 250 mV/S) at absolute temperature 298.15, 303.15 K represented in (Figure 7,8). The peak current [20,32-34] for both the anodic and cathodic peaks follows equation (6):
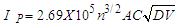 | (6) |
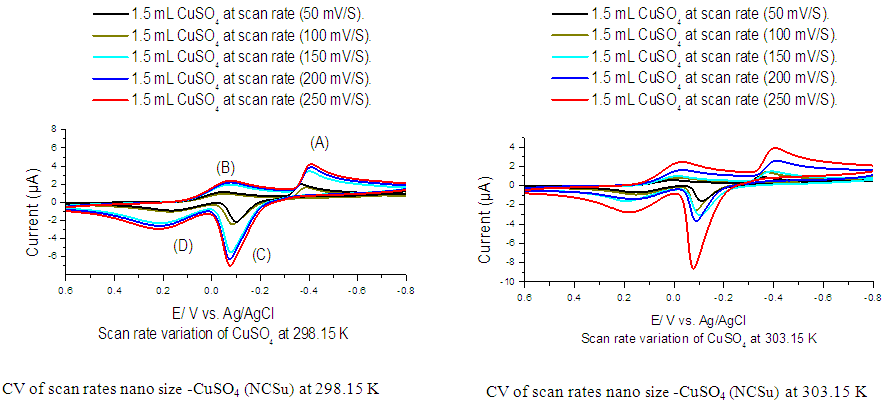 | Figure 7. Cyclic voltamogram of nano- CuSO4 (NCSu) in absence of Cefdinir (CED) antibiotic in 0.1 M KCl at 298.15, 303.15 K and different scan rates (50, 100, 150, 200 and 250 mV/S) |
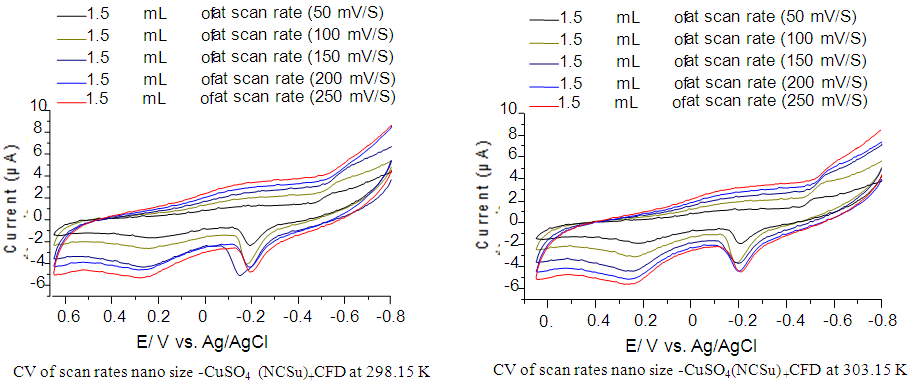 | Figure 8. Cyclic voltamogram of nano size-CuSO4 (NCSu) in the presence of Cefdinir (CED) antibiotic (1:1) in 0.1 M KCl at 298.15, 303.15 K and different scan rates (50, 100, 150, 200 and 250 mV/S) |
4. Conclusions
- The overall stability constants (β) and Gibbs free energies (ΔG) of complex formation for interaction of nano CuSO4 (NCSu) with Cefdinir (CED) antibiotic are slightly greater at 298.15K than that at 303.15K in 5% v/v Mixed DMF-H2O solvents indicating more interaction and complexation. The entropies and enthalpies for the interaction of nano CuSO4 (NCSu) + Cefdinir antibiotic (CED) are almost the same in the used two temperatures. This indicates the increment of temperature is followed by a decrease of complexation ability in this mixed solvents due to the migration of ions away from complexation field by increase of temperature as cyclic Voltammetry explained.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML