-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2020; 10(2): 25-31
doi:10.5923/j.materials.20201002.01
Received: Aug. 14, 2020; Accepted: Aug. 28, 2020; Published: Sep. 15, 2020

Anodically Deposited MnO2/Stainless Steel Supercapacitor Electrode at Different Mass Loadings and Different Na2SO4 Electrolyte Concentrations
Sameh Hassan, A. H. Khafagy, Dalia Usama
Physics Department, Faculty of Science, Menoufia University, Shebin El-Koom, Menoufia, Egypt
Correspondence to: Sameh Hassan, Physics Department, Faculty of Science, Menoufia University, Shebin El-Koom, Menoufia, Egypt.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Manganese dioxide thin films are prepared by anodic potentiostatic electrochemical deposition on etched stainless-steel substrates as a single supercapacitor electrode. Effects of different mass loadings of 25, 50, 100 µg/cm2 for manganese dioxide films deposited on stainless-steel current collector, and concentrations of Na2SO4 electrolyte solution in the range, from 0.1 to 0.9 mole/L, on the specific capacitance of the developed electrode are investigated using the cyclic voltammetry, galvanostatic charging-discharging curves, and electrochemical impedance spectra. The highest specific capacitances (484.7, 483.4 and 481.1 F/g) are obtained at 20 A/g (or 0.5 mA/cm2) with the electrode having mass loading of 25 µg/cm2 at 0.1, 0.3 and 0.7 mole/L of Na2SO4 electrolyte concentrations, respectively. This paper gives new vision on the charge storage mechanism in manganese dioxide/stainless-steel film as an active supercapacitor electrode material, and its transition between the pseudo-capacitive and double layer behaviors as an effect to the mass loading of the manganese dioxide film, and Na2SO4 electrolyte concentration.
Keywords: Electrolyte concentration, MnO2 film, Mass loading, Double layer, Pseudo-capacitive
Cite this paper: Sameh Hassan, A. H. Khafagy, Dalia Usama, Anodically Deposited MnO2/Stainless Steel Supercapacitor Electrode at Different Mass Loadings and Different Na2SO4 Electrolyte Concentrations, American Journal of Materials Science, Vol. 10 No. 2, 2020, pp. 25-31. doi: 10.5923/j.materials.20201002.01.
Article Outline
1. Introduction
- Supercapacitors are charge storage devices that received a lot of attention due to their high power density, excellent reversibility, and very long cycle life. Also, they deliver higher energy density than conventional capacitors and higher power density than batteries [1-3]. In the last decades, MnO2 has attracted great attention in the field of supercapacitor energy storage applications due to its abundance and untoxicity as compared with the toxic and expensive ruthenium oxide. The energy storage of MnO2 is attributed to ion insertion/desertion within its surface depending on particle size, surface area and porosity [4-6]. MnO2, among the other transition metal oxides, is the most widely investigated for pseudocapacitors due to its high theoretical specific capacitance of 1370 F/g, relatively low cost, and environmental friendly nature [7-12]. Also, MnO2 has been prepared by several different methods like, chemical precipitate [13], sol-gel [14], pyrogenation [15], mechanical grinding [16], hydrothermal synthesis [17], and electrochemical deposition [18]. It has been reported [17-20] that the electrochemical deposition method proved to be more effective for preparing MnO2 nanostructures. Electrochemical deposition of MnO2 could be prepared via two approaches; anodic oxidation and cathodic reduction, where the cations Mn2+ and anions MnO4- (Mn7+) precursors are commonly used in them, respectively. Both of anodic oxidation and cathodic reduction can be manipulated by potentiostatic (PS) at constant potential as well as galvanostatic (GS) at constant current processes [17,21-25]. It is worth mentioning that both (PS) and (GS) techniques could be electrochemically employed to produce nanostructures as well as amorphous films of MnO2 films. In this work, the charge storage mechanism and its transition between pseudo-capacitive behavior and double layer behavior have been shown by studying the effects of different mass loadings, and concentration of Na2SO4 electrolyte solution on the specific capacitance of single manganese dioxide psedocapacitor electrode that are investigated using: the cyclic voltammetry, galvanostatic charging/discharging curves, and electrochemical impedance spectra (EIS) methods to get optimized electrode for supercapacitor application.
2. Experimental
- Manganese dioxide films were electroplated anodically onto 2 cm2 of 304-stainless-Steel (SS) substrates of thickness 0.175 mm as a working electrode in 0.25 mole/L manganese acetate tetrahydrate (CH3COO)2 Mn.4H2O plating solution by potentiostatic (PS) method at 1 Volt at room temperature (29°C). The substrates were first etched in conc. H2SO4 98% for 30 minutes, and then rinsed thoroughly with distilled water and air dried. The mass loading of the electrode films was selected to be 25, 50 and 100 μg/cm2, and controlled by adjusting the total charge passed through the electrode during deposition process.The electrochemical characterization of the electrodes was performed by using the conventional three electrode system through EC-Lab software of SP-150 potentiostat/galvanostat device in an electrochemical cell with MnO2/stainless steel substrate as a working electrode, Ag/AgCl (KCl saturated) as a reference electrode, and platinum wire as a counter electrode. The prepared films were tested as electrodes for supercapacitor in different concentrations of Na2SO4 electrolyte 0.1, 0.3, 0.5, 0.7 and 0.9 mole/L (mole/L is abbreviated to M) using cyclic voltammetry (CV), galvanostatice charge-discharge (CD), and electrochemical impedance spectroscopy (EIS) measurements. CV tests were conducted in the potential range (0-1 V) with different scan rates of 10-100 mV/s, as well as, at different current densities ranging from 0.5-5 mA/cm2 in a voltage window between 0 and 1 V for CD tests. Besides, EIS data were recorded using sine wave voltage of amplitude 10 mV and frequency range of 100 kHz-10 mHz.
3. Results and Discussion
3.1. Galvanostatic Charge-Discharge
- Galvanostatic charge-discharge is a reliable method for specific capacitance determination of supercapacitor electrodes by getting almost an exact value for the practical application. The electrochemical performance of the prepared electrodes was first characterized by galvanostatic charge–discharge curves using chronopotentiometry at actual charging currents from 1 - 10 mA with potential window between 0 and 1 Volt, for each concentration of Na2SO4 electrolyte (0.1, 0.3, 0.5, 0.7 and 0.9 mole/L), and for the different mass loadings of MnO2 films (25, 50 and 100 μg/cm2). Galvanostatic CD curves for 25 μg/cm2 deposited mass of MnO2 electrodes are shown in Figure 1, measured at actual current 1 mA in different Na2SO4 electrolyte concentrations as indicated.
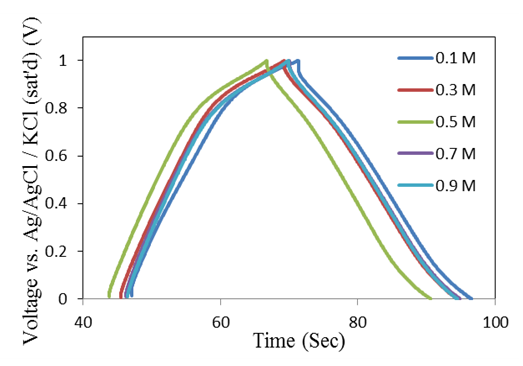 | Figure 1. Galvanostatic CD curves for deposited MnO2 electrodes at mass loading of 25 μg/cm2, measured at actual current 1 mA in different Na2SO4 electrolyte concentrations |
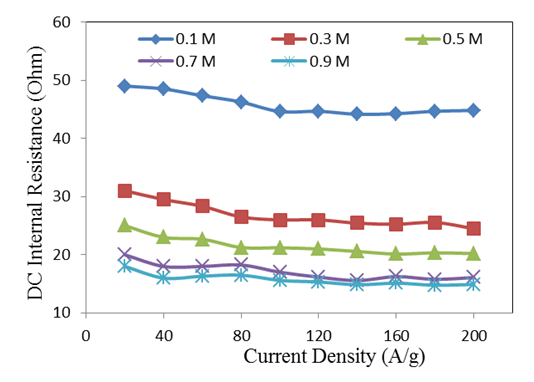 | Figure 2. Variation of DC internal electrode resistance with current densities for electrode mass loading of 25 μg/cm2 |
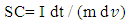 | (1) |
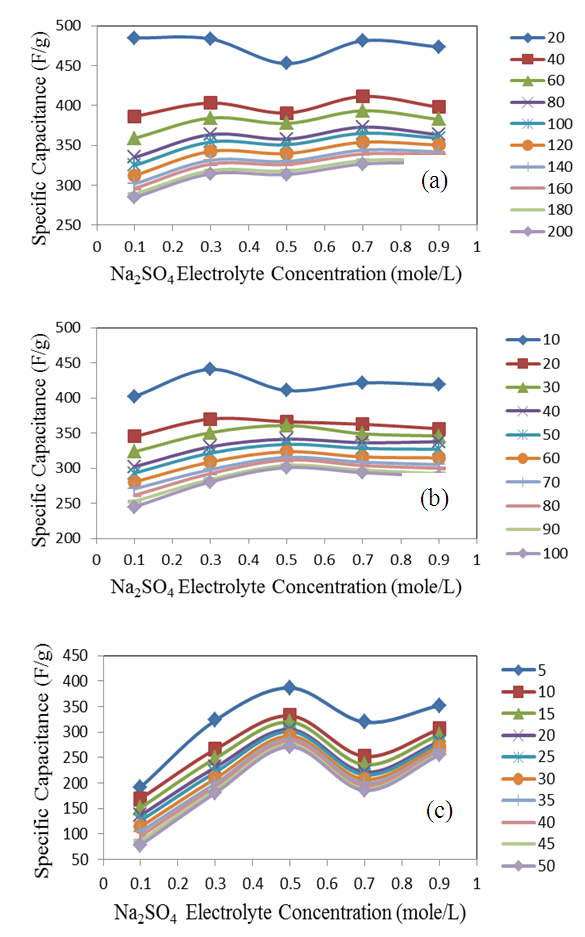
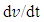 is the slope of the discharge half cycle.Figure 3 shows the dependence of specific capacitance on current density for MnO2 deposited films of mass loading 25 μg/cm2; as measured in different investigated electrolyte concentrations. From inspection of this Figure, it was observed that the SC gradually decreases as the current density increases for all cases of investigated electrolyte concentrations keeping the level of SC values associated to lower concentration solution, 0.1 M is lower than that one’s of higher concentrations especially at higher current density. This common decrease behavior can be attributed to the high accessibility of ions to the pores of electrode film surface at low charging rates. The highest specific capacitance value obtained for mass loading of 25 μg/cm2 at 1 mA is 484.7 F/g at the corresponding Na2SO4 electrolyte concentrations 0.1 mole/ L, while that obtained for mass loading of 50 μg/cm2 is 440.7 F/g at Na2SO4 electrolyte concentrations 0.3 mole/L, and for mass loading of 100 μg/cm2 is 386.7 F/g at Na2SO4 electrolyte concentrations 0.5 mole/L.
is the slope of the discharge half cycle.Figure 3 shows the dependence of specific capacitance on current density for MnO2 deposited films of mass loading 25 μg/cm2; as measured in different investigated electrolyte concentrations. From inspection of this Figure, it was observed that the SC gradually decreases as the current density increases for all cases of investigated electrolyte concentrations keeping the level of SC values associated to lower concentration solution, 0.1 M is lower than that one’s of higher concentrations especially at higher current density. This common decrease behavior can be attributed to the high accessibility of ions to the pores of electrode film surface at low charging rates. The highest specific capacitance value obtained for mass loading of 25 μg/cm2 at 1 mA is 484.7 F/g at the corresponding Na2SO4 electrolyte concentrations 0.1 mole/ L, while that obtained for mass loading of 50 μg/cm2 is 440.7 F/g at Na2SO4 electrolyte concentrations 0.3 mole/L, and for mass loading of 100 μg/cm2 is 386.7 F/g at Na2SO4 electrolyte concentrations 0.5 mole/L.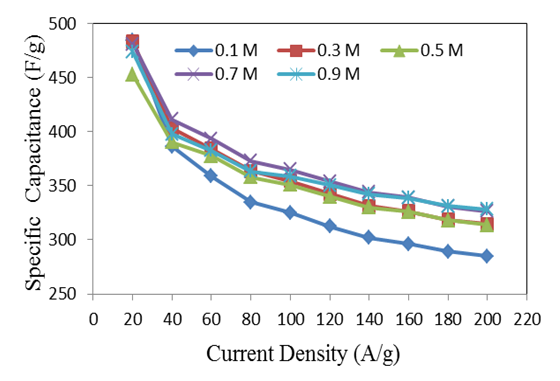 | Figure 3. Variation of specific capacitance with current density for different Na2SO4 electrolyte concentration at mass loading of electrode film 25 μg/cm2 |
3.2. Cyclic Voltammetry (CV)
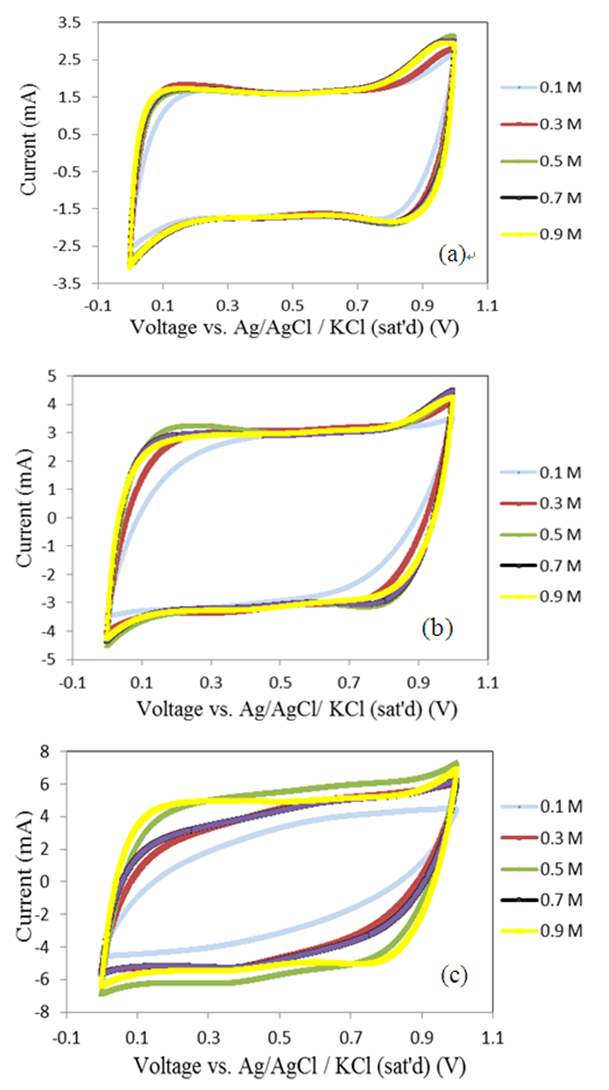
- The cyclic voltammetry curves (CV) of potentiostatic anodically deposited MnO2 films at different mass loadings 25, 50, and 100 µg/cm2 on etched SS electrodes were investigated in Na2SO4 electrolyte solution of different concentrations 0.1, 0.3, 0.5, 0.7, and 0.9 mole/L at different applied voltage scan rates, from 10 mV/s to 100 mV/s, in the potential window range, from 0 to 1 V for each concentration. Figure 5(a)-(c) shows the CV plots at scan rate of 100 mV/s for tested concentrations at different mass loadings (a) 25, (b) 50, and (c) 100 μg/cm2. Those curves indicate that rectangular curves without redox peaks are obtained in the tested potential window, indicating the existing of a high capacitive behavior with a good response of ion (or charge carrier) transfer. However, the resistive like behavior obtained at high mass loading of 100 µg/cm2 in low electrolyte concentration of 0.1 mole/L at scan rate of 100 mV/s, is related to the high resistance due to mass increase and decreasing number of electrolyte ions which is a characteristic behavior of high mass loading of thin films.
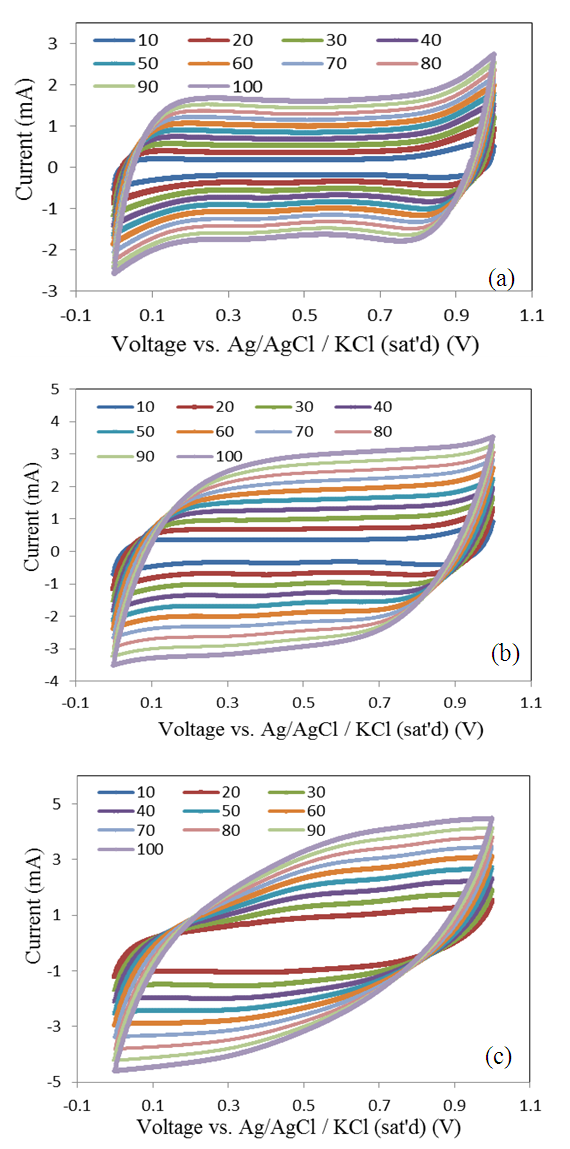 | Figure 6. CV curves for deposited MnO2 films at different mass loadings (a) 25 (b) 50, and (c) 100 μg/cm2 at different scan rates (10-100 mV/s) in 0.1 mole/L concentration of Na2SO4 electrolyte |
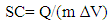 | (2) |
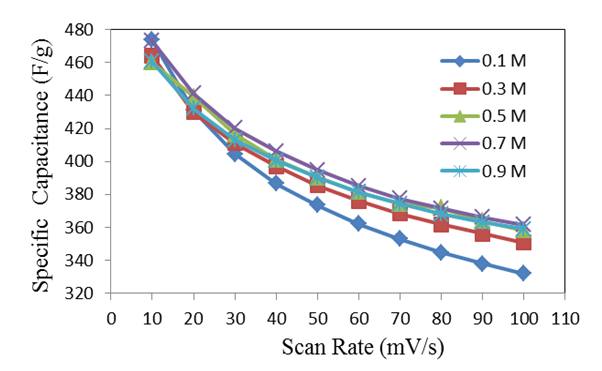 | Figure 7. The variation of specific capacitance with scan rate for deposited MnO2 film at mass loading of 25 µg/cm2, as measured in different electrolyte concentrations of Na2SO4 |
3.3. Impedance Spectroscopy Technique
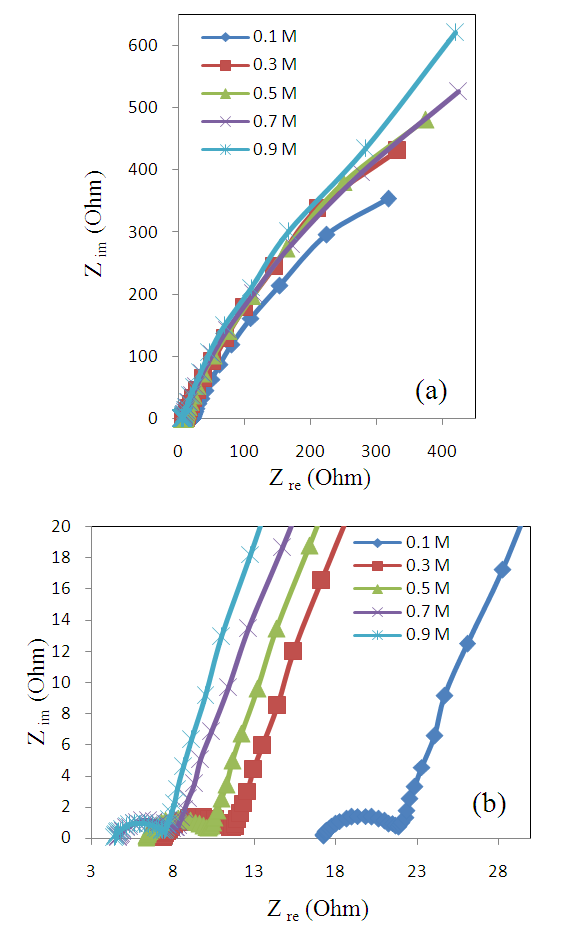
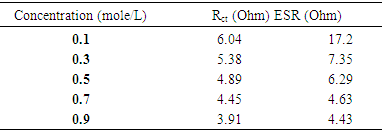
- In general, the power output capability of electrochemical supercapacitors, depend strongly on the rates of ionic mass transport and the equivalent series resistance (ESR) [30,31]. Electrochemical impedance spectroscopy (EIS) has been widely used to study the redox (charging/discharging) processes of electrode materials and to evaluate their electronic and ionic conductivities.Figure 8(a) shows the obtained results of electrochemical impedance spectroscopy measurements, recorded in the frequency range of 10 mHz–100 kHz, under applying 10 mV amplitude at room temperature for the deposited mass 25 μg/cm2 of MnO2 film. While Figure 8(b) represents a part of Figure 8(a) which is corresponding to lower values of both real and imaginary impedances of Figure 8(a) after being zoomed in.
|
4. Conclusions
- Three different mass loadings (25, 50 and 100 μg/cm2) of MnO2 films were anodically electrodeposited from 0.25 M manganese acetate solution by applying potentiostatic technique on 304-stainless steel current collector etched with 98% H2SO4. Effect of concentration of Na2SO4 electrolyte solution (ranged from 0.1 to 0.9 M) on the capacitive behavior was studied and optimized. According to the electrochemical measurements, it was found that the highest specific capacitances (484.7, 483.4 and 481.1 F/g) was obtained at current density of 20 A/g (or 0.5 mA/cm2) for MnO2/SS electrode with 25 µg/cm2 mass loading at 0.1, 0.3 and 0.7 mole/L Na2SO4 electrolyte concentration, respectively. This optimized capacitive behavior with the potentiostatically deposited MnO2 film on 304-stainless steel current collector can be considered as a promising electrode for high energy storage supercapacitors.
ACKNOWLEDGEMENTS
- This work was financially supported by Science & Technology Development Fund (STDF), Egypt, Grant No 13855.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML