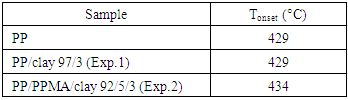-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2020; 10(1): 15-24
doi:10.5923/j.materials.20201001.03
Received: July 2, 2020; Accepted: July 27, 2020; Published: August 15, 2020

Development of Polypropylene Nanocomposites Filled with Clay Particles from Cubati/Brazil
Carlos I. R. de Oliveira 1, Marisa C. G. Rocha 1, Joaquim T. de Assis 1, Nancy I. Alvarez Acevedo 1, Ana L. N. da Silva 2, Luiz C. Bertolino 3
1Instituto Politécnico- IPRJ, Universidade do Estado do Rio de Janeiro - UERJ, Nova Friburgo, Brazil
2Instituto de Macromoléculas Professora Eloisa Mano - IMA, Universidade Federal do Rio de Janeiro – UFRJ, Rio de Janeiro, Brazil
3Centro de Tecnologia Mineral - CETEM, Ministério da Ciência, Tecnologia e Inovações - MCTI, Rio de Janeiro, Brazil
Correspondence to: Marisa C. G. Rocha , Instituto Politécnico- IPRJ, Universidade do Estado do Rio de Janeiro - UERJ, Nova Friburgo, Brazil.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of this work is to expand the field of applications of bentonite clays from Cubati, Paraíba, through the development of polymeric nanocomposites. A Brazilian bentonite clay from Cubati, Paraíba, was used to produce an organophilic clay. The pristine and modified clays were characterized by infrared spectroscopy (FTIR), X-ray fluorescence (XRF) and X-ray diffraction (XRD). The results indicated that the clay was organophilized. The PP/clay nanocomposites were prepared with 3 wt% of organoclay and 5 wt% of a polypropylene grafted maleic anhydride (PP-g-MA) in a co-rotating twin-screw extruder. The produced materials were characterized by XRD, transmission electron microscopy (TEM), differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). The tensile properties and impact strength were also determined. The results indicated that PP/PPMA/clay nanocomposite presents higher thermal stability and crystallization temperature than the PP matrix. The PP’s Young’s modulus and tensile strength were not significantly affected. However, there was a significant increase of the elongation at break. These findings show that Brazilian bentonite clay can used as a filler in PP nanocomposites.
Keywords: Compatibilizer, Characterization, Nanocomposites, Organophilic bentonite, Polypropylene
Cite this paper: Carlos I. R. de Oliveira , Marisa C. G. Rocha , Joaquim T. de Assis , Nancy I. Alvarez Acevedo , Ana L. N. da Silva , Luiz C. Bertolino , Development of Polypropylene Nanocomposites Filled with Clay Particles from Cubati/Brazil, American Journal of Materials Science, Vol. 10 No. 1, 2020, pp. 15-24. doi: 10.5923/j.materials.20201001.03.
Article Outline
1. Introduction
- Polymer/layered silicate (clay) nanocomposites have been widely studied due to the many advantages these materials present compared to conventional composites [1,2]. The polymer/clay nanocomposites comprise a class of materials in which the inorganic phase is dispersed in the polymer matrix at nanometer level. The main difference between nanocomposites and traditional composites is that processing of nanocomposites requires low filler contents (up to 5% by weight), while traditional composites need high content of filler, up to 40 wt% [3]. Nanocomposites began to be studied in the 1980s by the Toyota Research Laboratory, with the development of nanocomposites of polyamide and clay [4]. Polymer/clay nanocomposites have superior mechanical and thermal properties, flame retardancy and dimensional stability and lower permeability to gases than those presented by the conventional composites [5-7]. Because of this, there are numerous studies on these materiais [8-10].Among the different clays currently used for the preparation of polymeric nanocomposites, the bentonite clays have long been used, mainly because of their attractive properties, such as very high aspect ratio and surface area. Besides that, these materials are environmentally friendly and available in large quantities. In Brazil, there are large deposits of clays [3,11,12]. Bentonite rocks are composed essentially of one or more minerals (mainly montmorillonite) of the smectite clay group, and the physical properties of the clays are mainly established by this mineral [13,14]. Bentonite is an aluminosilicate 2:1, [(Mg,Ca)O.Al2O3Si5O10.nH2O)], formed from the decomposition of volcanic ash, which in its natural form has the exchangeable cations Na+, Mg2+, Ca2+, Al3+ and Fe3+. In Brazil, the most common form of this occurrence is as a polycationic bentonite [11]. Bentonites are very fine-grained clays, with high adsorption capacity, high content of colloidal matter and high possibility of activation. Most Brazilian bentonite reserves, about 62%, are located in the state of Paraiba, in the municipalities of Boa Vista and Cubati. The state of São Paulo accounts for 28% of the reserves and the states of Bahia, Minas Gerais and Paraná account for the remaining 10% [3]. Although, there are many published studies on clays from Boa Vista, there are few studies on nanocomposite production from Cubati clays [15]. The industrial application of clays requires a complete identification of its nature and properties. Depending on the deposit from which the clay was extracted, there are may be some difference in properties, even for a given clay variety [16]. The reserves in Boa Vista are depleted, it is urgent to assess clays with potential from other deposits in Paraiba, such as Cubati.Despite the numerous advantages of using bentonite clays to prepare polymer nanocomposites, the natural hydrophilicity of these silicates hinders the interaction and the ability to blend them with non-polar polymeric matrices [8,17]. To improve interaction with the polymer, the clay must be organically modified, giving rise to an organoclay. Among the different processes used to obtain organophilic bentonite clays, the use of quaternary ammonium salts containing chains of different structures is one of the most widely used methods [17]. The presence of organic cations between bentonite clay layers decreases the surface tension of these clays and improves the compatibility with polymeric matrices [18]. The bentonite clays are the most used in the preparation of organophilic clays due to the small size of the crystals, the high cation exchange capacity (CEC), large surface area and good swelling capacity in water [19]. This set of properties allows the rapid intercalation of organic compounds and a reaction yield of 100% [20,21]. Polypropylene (PP) is one of the most widely used non-polar polymers due to its low cost, low density and the high specific properties. In recent years, PP/clay nanocomposites have attracted strong research interest [22-24]. However, the absence of polarity of the PP hinders interaction and intercalation or exfoliation of the polymeric chains between the platelets of the clay particles [25]. Consequently, the matrix modification with polar moieties is necessary prior to modified clay introduction in order to achieve nanometric dispersion of the clay [26]. Several studies have been performed to evaluate the use of polypropylene grafted with maleic anhydride and acrylic acid as compatibilizers in polypropylene/organoclay nanocomposites [26,27].According to López-Quintanilla et al. (2006) in the melt process of obtaining nanocomposites, the exfoliation and dispersion of nanoclays in polypropylene is a function of some parameters such as: type of clay and its pretreatment method, concentration of functional groups in the compatibilizer and its overall concentration in the composite, the viscosity of the polymer, and the operational conditions [26].The aim of this work is to expand the field of applications of bentonite clays from Cubati through the development of polymeric nanocomposites. For this purpose, in this work, polypropylene/clay nanocomposites were prepared and their properties were determined. The efficiency of polypropylene grafted with maleic anhydride (PP-g-MA) as a compatibilizing agent was also evaluated.
2. Experimental
2.1. Materials
- Polypropylene homopolymer (PP, H501HC) with a melt flow index (MFI) of 3.5 g/10 min (230°C/2.16 kg), supplied by Braskem S.A., was used. Polypropylene grafted with maleic anhydride (PP-g-MA), Polybond 3200, with a melt flow index (MFI) of 109 g/10 min (190°C/2,16 Kg) and having 2.7 wt% MA content was supplied by Chemtura Indústria Química do Brasil Ltda.Bentonite clay from Cubati, Paraíba, a municipality located in the northeastern region of Brazil (geographical coordinates 24M 0799950 9245922), was used. The clay sample was sifted by passing it through ABNT 635 mesh (0.022 mm) and identified according to its source as JG clay. The quaternary ammonium salt, cetyltrimethyl ammonium chloride, commercially known as Dehyquart® A, from Farmos Química Fina, was used to modify the bentonite.
2.2. Preparation of Organoclay
- To make the interlayer spacing more homogeneous and regular, the JG clay was treated with a solution of sodium chloride. In this treatment, 100 g of JG clay, 500 g of NaCl and 1000 ml of deionized water were placed in a glass reactor equipped with a mechanical stirrer. The system was heated in a water bath at 55°C and kept under stirring for 72 hours. Subsequently, the product was centrifuged with deionized water (2000 ml) for 45 minutes at 2000 rpm to remove the Cl- anions. The absence of Cl- anions was confirmed by titration with 0.1 M AgNO3. Then the homoionic clay was dried at 60°C for 48 h in an oven with air circulation, ground and screened with a 200 mesh sieve.To obtain the organophilic clay using the quaternary ammonium salt (cetyltrimethyl ammonium chloride, a dispersion containing 60 g of sodium clay, 72 g of quaternary ammonium salt and 1200 ml of deionized water was prepared in a glass reactor equipped with a mechanical stirrer. The system was heated in a water bath at 55°C and kept under stirring for 72 hours. Subsequently, the product was centrifuged with deionized water (2000 ml) for 45 minutes at 2000 rpm to remove the Cl- anions. The absence of Cl- anions was confirmed by titration with 0.1 M AgNO3. The organophilic clay was dried at 60°C for 48 h in an oven with air circulation, ground and screened with a 200 mesh sieve and stored for further characterization.
2.3. Preparation of Nanocomposites
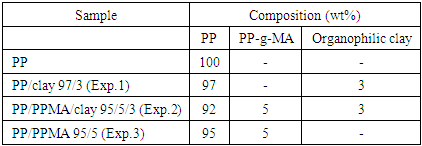
- Prior to blending, PP and organoclay were oven dried at 60°C for 24 h. The PP-g-MA was placed in a desiccator for 24 h before blending. These components were physically mixed and then extruded in a Teck Tril model DCT 20 co-rotating twin-screw extruder (L/D ratio of 36). The extrusion was performed with a temperature profile equal to 90/120/160/180/180/190/190/200/200/210°C (from the feed to the die zone). The screw speed was set at 500 rpm. The nanocomposites were prepared by dispersing the modified bentonite (3 wt%) into the polypropylene matrix without and with 5 wt% of PP-g-MA. The pellets obtained were injection molded in an Arburg model Allrounder 270 S molding machine with a temperature profile of 210/200/190/180/170°C. The test specimens’ dimensions corresponded to those specified in ASTM D638 (tensile test) and ASTM D256 (impact test). The sample formulations and codes are summarized in Table 1.
|
2.4. Characterization of Clays and Nanocomposites
- The infrared analysis was performed on the clay samples using a Varian Excalibur 3100 spectrometer. The spectra were obtained in the 4000 to 400 cm-1 range, with samples pressed as KBr pellets.The chemical composition of the clays was determined by X-ray fluorescence (XRF) in a WDX fluorescence spectrometer, Axios model (Panalytical). The semi quantitative results were calculated as oxides and normalized to 100%. The loss on ignition (LOI) was determined according to LOI = (Wd – Wf)/Wd x 100, where Wd is the weight of the dry sample at 110°C and Wf is the weight of the calcined sample at 1000°C during 3 hours.XRD measurements of clays were performed on a sample in the form of powder with an Ultima IV X-ray diffractometer (Rigaku Corporation) using a potential difference of 40 kV and tube electric current of 20 mA. The scan was performed in the angular region 0.9 to 10° (2θ) at 1° (2θ)/min. The radiation used was CuKα with λ = 1.5418 Å. For analysis of nanocomposites, 0.2-mm thick films were compression molded by heating at 190°C under 69 MPa for 15 min, and cooling for 5 min in a cold press. The interplanar distance (d) was calculated based on the results, according to Bragg’s law.The thermal stability of the nanocomposites was evaluated by thermogravimetric analysis (TGA) in a TA Instruments model Q500 analyzer. The samples were heated from 30 up to 700°C, at a heating rate of 10°C/min under nitrogen flow.The thermal behavior of the materials was analyzed in a TA Instruments Q1000 differential scanning calorimeter (DSC). The samples were heated to 200°C, kept at this temperature for 5 min, and then cooled to room temperature at 10°C/min. They were then reheated to 200°C at same heating rate. The crystallization and melting thermograms were recorded from the cooling and second heating cycles, respectively. The melting temperature (Tm) and crystallization temperature (Tc) were determined from the DSC diagrams. Sample crystallinity content was calculated using a PP fusion enthalpy reference value of 190 J/g [28].The clay dispersion of nanocomposite samples was examined by transmission electron microscopy (TEM). Ultra-thin sections (50 nm) were obtained with a Leica EM UC6 ultra-microtone and collected on a copper grid. TEM images were recorded with a FEI-Tecnai G2 Spirit with a LaB6 filament and acceleration voltage of 120 kV.The tensile properties of PP and PP/PP-g-MA/clay nanocomposites were determined using a Shimadzu AG-I universal testing machine with a 5 kN load cell. Tests were conducted according to ASTM D 638 using Type I test specimen dimensions (seven specimens for each test). A crosshead speed of 20 mm/min was employed. The Izod impact strength of notched specimens was determined in a CEAST model 9050 impact machine according to ASTM D256.
3. Results and Discussion
3.1. Characterization of Clays
3.1.1. X-ray Fluorescence (XRF)
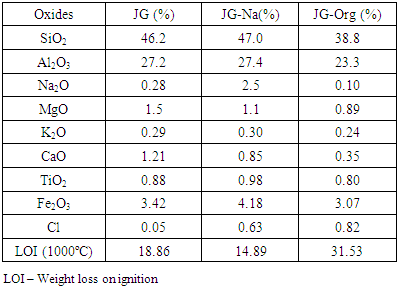
- Table 2 presents the results of chemical analysis obtained by X-ray fluorescence (XRF) for the pristine clay (JG), the homoionic clay (JG-Na), and the organically modified clay (JG-Org), prepared with cetyltrimethyl ammonium chloride.
|
3.1.2. Fourier-Transform Infrared Spectroscopy (FTIR)
- Figure 1 shows the FTIR spectra of JG, the homoionic (JG-Na), and organically modified (JG-Org) clays.All the samples showed absorption bands at 3698 and 3623 cm-1, attributed to the OH stretching, corresponding to structural hydroxyl groups of the clay. Absorption bands at 3400 and 1638 cm-1, attributed to the angular vibration of the OH group and related to the adsorbed water and the hydration water present in the clay, can also be seen in the spectra [30].Characteristic absorption of montmorillonite clay can be observed in the region between 1115 and 1034 cm-1, related to the Si-O bond, and at 915, 798 and 542 cm-1, corresponding to the octahedral layers of the aluminosilicate. No changes were found in the spectrum of sodium clay (JG-Na) (b) in relation to the pristine clay (JG) (a).
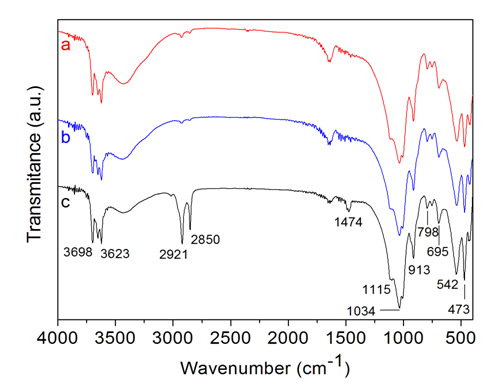 | Figure 1. FTIR spectra of the bentonite clays: (a) JG, (b) JG-Na and (c) JG-Org |
3.1.3. X-ray Diffraction (XRD)
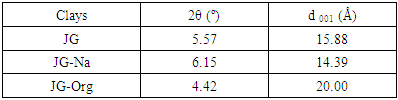
- Analyses by X-ray diffraction (XRD) were conducted in order to verify the possible intercalation of quaternary ammonium salt between the lamellae of JG clay. Figure 2 shows the diffractograms.
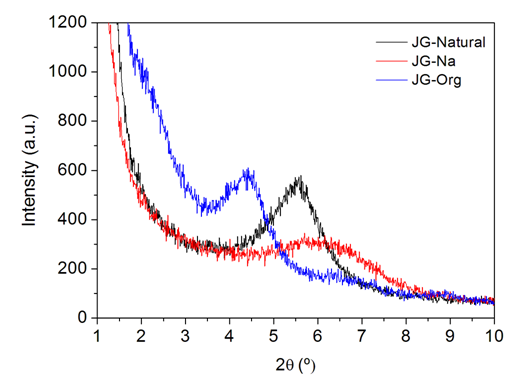 | Figure 2. Diffractograms of the bentonite clays |
|
3.2. Characterization of Nanocomposites
3.2.1. Structure and Morphology
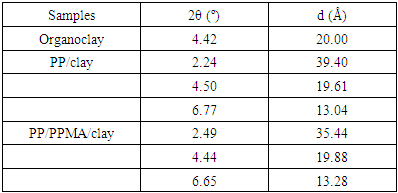
- The structure and morphology of the nanocomposites were evaluated by X-ray diffraction and transmission electron microscopy, respectively. X-ray diffraction patterns of the PP/clay and PP/PP-g-MA/clay nanocomposites as well as the organoclay (used as reference), in the region corresponding to 2θ values from 1° to 10° are presented in Figure 3.
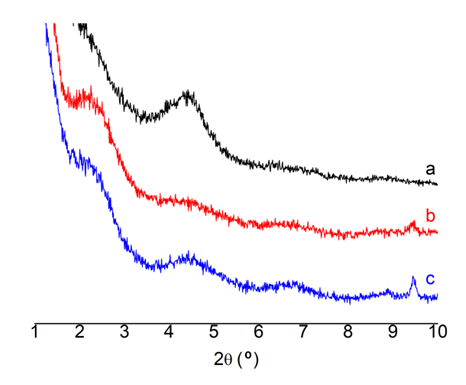 | Figure 3. XRD patterns of the organoclay (a), PP/clay (b) and PP/PPMA/clay (c) |
|
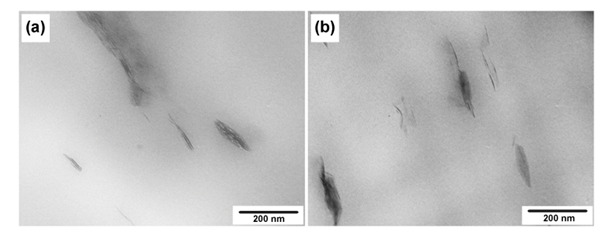 | Figure 4. TEM micrographs of PP/clay (a) and PP/PPMA/clay (b) |
3.2.2. Differential Scanning Calorimetry (DSC)
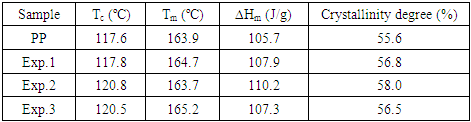
- To evaluate the thermal properties of the nanocomposites, non-isothermal crystallization DSC scans were carried out. Table 5 shows values for melting temperature (Tm), crystallization temperature (Tc), melting enthalpy (ΔHm) and crystallinity degree of PP, PP/clay, PP/PPMA/clay and PP/PPMA samples.
|
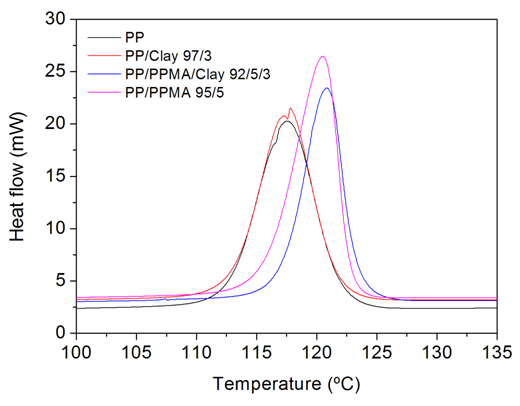 | Figure 5. Crystallization peaks of PP, PP/clay, PP/PPMA/clay and PP/PPMA samples |
3.2.3. Thermogravimetric Analysis (TGA)
- Determination of the thermal properties and stability of polymer clay nanocomposites is very important, mainly due to possible variations in the structure and degradation of the material from the processing conditions employed. At processing temperatures higher than the thermal stability of the organic salts used for modification of the clays, decomposition will take place [40]. In the present work, the processing temperatures were defined according to evaluation of the thermal stability of organically modified clay and processing conditions observed in the literature [41]. The thermogravimetric analyses were used to investigate the thermal stability of the PP/clay and PP/PPMA/clay nanocomposites. With this purpose, the degradation onset temperature (Tonset) was determined. The results obtained are reported in Table 6.
|
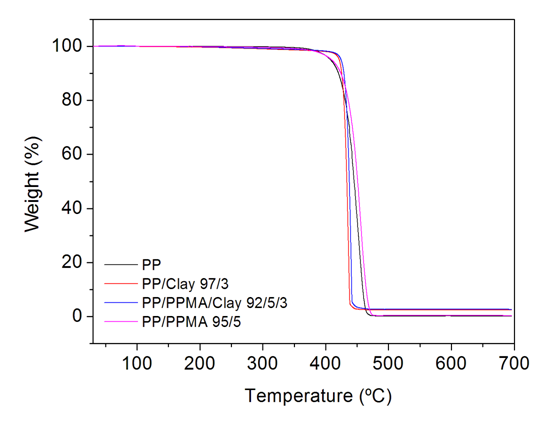 | Figure 6. Weight loss of PP/PPMA/clay nanocomposites as a function of temperature |
3.2.4. Tensile Properties
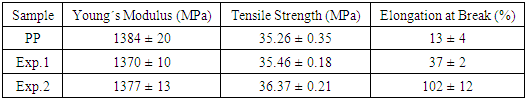
- The study of the mechanical properties of nanocomposites is very complex, since these properties are influenced by several variables, such as processing machinery [3,42]; processing conditions [42]; chemical nature of the clay surface modifier [42]; type and concentration of clay and compatibilizer [42-44]; compatibilizer/nanoclay ratio [44]; viscosity of the compatibilizer, and polypropylene melt flow rate (MFR) [33]. Therefore, several studies have been published showing a large improvement of the mechanical properties of PP/clay nanocomposites, while others have shown no noteworthy changes in the mechanical behavior of this polymer [3]. Table 7 presents the mechanical property data obtained in this work.
|
|
3.2.5. Impact Strength
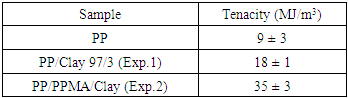
- The Figure 7 shows the Izod impact strength of polypropylene and the PP/clay nanocomposites. The results show that the addition of clay as well as the addition of clay and compatibilizer together decreased the impact strength of polypropylene. This result can be attributed to the partial dispersion of the clay in the PP matrix. It is not easy to predict the effect of nanosize particles on the impact strength of nanocomposites. Some researchers have reported that impact strength is strongly influenced by the content of coupling agent and also by the quality of the clay [41]. According to Chen et al. (2009), it is difficult to predict how nano-reinforcements will affect impact strength, because of the confounding effects of crystallinity, spherulite size, preferred orientation or processing variables [46].
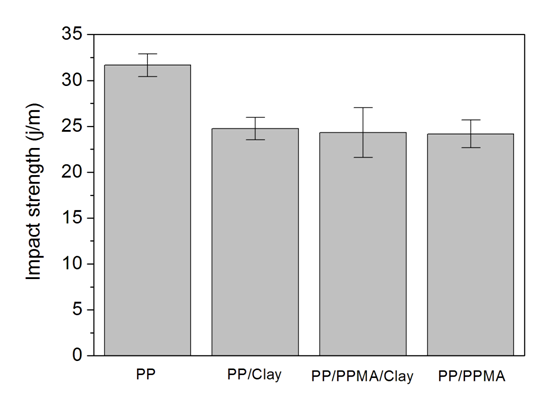 | Figure 7. Izod impact strength of PP, PP/clay and PP/PPMA/clay nanocomposites |
4. Conclusions
- The results obtained in this work indicate there was partial intercalation of polypropylene chains in the clays layers. The PP/PMMA/clay nanocomposite presented higher thermal stability and crystallization temperature than the PP matrix. The Young’s modulus and tensile strength of PP were not affected. However, there was a significant increase in the elongation at break. The impact strength decreased slightly with the addition of clay and compatibilizer. These findings show that Brazilian bentonite clay can be used as a filler in PP/nanocomposites. However, other experimental conditions should be evaluated to obtain better dispersion of clay in the polymer matrix and a larger improvement in the properties.
ACKNOWLEDGEMENTS
- This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. We also thank Conselho Nacional para o Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) for financial support and Centro de Tecnologia Mineral (CETEM) for supplying the clay samples.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML