-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2020; 10(1): 1-8
doi:10.5923/j.materials.20201001.01

Synthesis of Cellulose-Based Superabsorbent Hydrogel from Rice Husk Using a Microwave
Adamu Abdulhameed, Harun M. Mbuvi, Evans O. Changamu
Chemistry Department, Kenyatta University, Nairobi, Kenya
Correspondence to: Harun M. Mbuvi, Chemistry Department, Kenyatta University, Nairobi, Kenya.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The present work focuses on the synthesis of a new environmentally friendly superabsorbent hydrogel derived from a cellulose derivative using 1, 2-ethanediol (glycol) as crosslinking agent. The cellulose isolated from rice husk, which has a basis to modify and obtain carboxymethylcellulose CMC using sodium hydroxide (NaOH) and monochloroacetic acid (MCA). Fourier transform infrared (FTIR) and X-ray diffraction (XRD) were performed in other to investigate the reactivity of the superabsorbent hydrogel. The Optimum conditions of power, time, CMC dose and amount of cross-linker required for the production of most desirable, stable and high water absorptivity were investigated, the optimum swelling capacity was found to be 1162%.
Keywords: Cellulose, Superabsorbent hydrogel, Crosslinking, Microwave
Cite this paper: Adamu Abdulhameed, Harun M. Mbuvi, Evans O. Changamu, Synthesis of Cellulose-Based Superabsorbent Hydrogel from Rice Husk Using a Microwave, American Journal of Materials Science, Vol. 10 No. 1, 2020, pp. 1-8. doi: 10.5923/j.materials.20201001.01.
Article Outline
1. Introduction
- Superabsorbent hydrogels are 3-dimensional hydrophilic (water-loving) polymers, which have the ability of absorbing and retaining a high amount of water, and the releasing it under stress conditions. The ability of the hydrogel to absorb water is as a result of the hydrophilic functional groups (OH, CONH, CONH2, COOH, and SO3H) attached to the polymer backbone while their resistance to dissolution arises from the cross-linker between networks (Raju et al., 2003). The network structure of the polymers can be affected by environmental factors such as temperature, pH and ionic strength of the aqueous solution (Tanaka, 1978). Literature has shown that most of the available superabsorbent hydrogels are derived synthetically and most are known to be toxic to the environment due to non-degradability and non-biocompatibility (Shang et al., 2008). There is need to develop superabsorbent hydrogels that are eco-friendly, especially natural materials such as cellulose, starch and chitosan. Consequently, they have attracted much attention due to their cost-effective, degradability and availability (Mahmoudian and Ganji, 2017). Superabsorbent hydrogels are widely used in various fields such as hygiene napkins, disposal diapers, soil for horticulture and agriculture, water and food purification, (Wang et al., 2008). Nowadays, researchers have a focus on the development of hydrogels for tissue engineering (Khan et al., 2009), sensor (Sorber et al., 2008) and drug delivery (Wu et al., 2008).Cellulose is the most abundant renewable, biocompatible and degradable linear, long-chain, water-insoluble, natural polymer on earth. In this study, cellulose was extracted from rice husk, and modified to carboxymethylcellulose (CMC). Carboxymethylcellulose can be obtained when cellulose is reacted with monochloroacetic acid (MCA) or its sodium salt under alkaline condition in the presence of an organic solvent (Putri and Kurniyata, 2016). The OH groups of cellulose at C2, C3, and C6 are substituted by sodium carboxymethyl group, depending on the degree of substitution. Several studies have been done on synthesis of cellulose based superabsorbent hydrogel by using Carboxymethylcellulose cross-linked carbodiimide (Sannino et al., 2006) and divinylsulphone (Sannino and Nicholas, 2005). However, these cross-linkers were reported to be toxic, expensive and unavailable. Subsequently, to overcome such, citric acid (which is the reverse) was develop as a new crosslinking agent (Dimitri et al., 2008). In regard to that, not much has been done on the crosslinking of Carboxymethylcellulose (CMC) with 1, 2-ethanediol (glycol) using a microwave. Sodium carboxylmethylcellulose (CMC) once linked with 1,2-ethanediol, an ester linkage will be formed as shown in figure 1. When in a dry powder state, the chains of the polymers twisted and lined with carboxyl group or (-COOH). When placed into water, it breaks apart into positive and negative ions as common salt. When hydrated with water, the carboxyl group dissociates into charged carboxylate ions (COO-). These ions repel each other on the polymer chain thereby widening the polymer coils permitting water to penetrate and contact with a lot of carboxyl group.
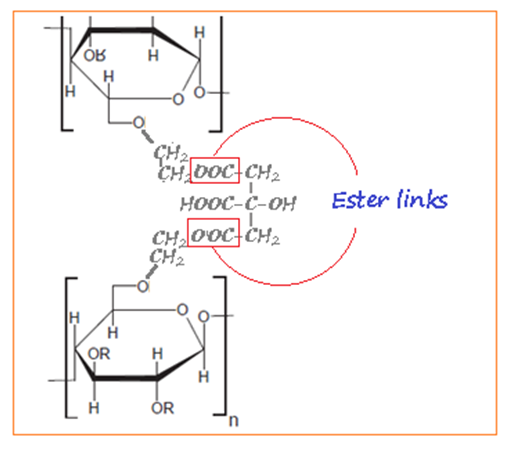 | Figure 1. Formation of the ester linkage |
2. Materials and Methods
2.1. Materials
- The rice husk was obtained from Euro rice mills in Mwea, Kirinyaga County – Kenya. Sodium hydroxide and glacial acetic acid were prepared from Merck Chemical Co. (Darmstadt, Germany). Nitric acid, Ethanol and methanol were provided from the local market. Monochloroacetic acid (MCA) was purchased from Daejung Co. (South Korea) and an RM 240 microwave with a maximum power output of 700 watts was used in the synthesis process.
2.2. Methods
2.2.1. Extraction of Cellulose
- About 5.00g of rice husk was weight in 250mL Erlenmeyer flask and 100mL of 80% glacial acetic acid, 10mL of 70% nitric acid was added. The flask was covered using aluminium foil and heated in an oven at 120°C for 20 minutes. The sample mixture was allowed to cool and 60mL of distilled water was added, the mixture was filtered and washed with distilled water and 95% ethanol. The residue was dried in an oven at 60°C for 19hrs. (Brendel et al., 2010)The extract yield of cellulose was calculated using the formula below
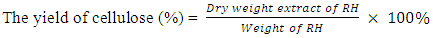 | (1) |
2.2.2. Synthesis of Carboxymethylcellulose (CMC)
- The synthesis of carboxymethylcellulose (CMC) involves two stages: alkalization and carboxymethylation (etherification). About 5.00g of the extracted cellulose was added to 100mL of distilled water in a 250mL Erlenmeyer flask. Then 10mL of 30% of sodium hydroxide solution was added dropwise. The alkalization process was carried out for 1hr at 25°C on a fitted shaker. Then 5.00g of monochloroacetic acid were added to the mixture and heated in a microwave at the power of 6 for 2 minutes, the mixture was filtered. Neutralization obtained residue by soaking with 100mL of methanol for 24hrs, then the mixture was neutralized using glacial acetic acid. The mixture was filtered again and the residue was dried in an oven at 60°C.The yield of carboxymethylcellulose was calculated using the formula,
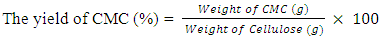 | (2) |
2.2.3. Synthesis of Superabsorbent Hydrogel
- About 2.00g of Carboxymethylcellulose (DS-0.79) was dissolved in distilled water by stirring at room temperature using a magnetic stirrer for 24 hrs, after obtaining a solution, a variable amount of 1, 2-ethanediol was added as the cross-linker. The solution was heated in a microwave at various power outputs and time. To remove the unreacted chemicals, the contents were washed several times with distilled water until the hydrogel was perfectly transparent. It was then dried in an oven at 45°C until it maintains a constant weight.
2.2.4. Swelling Ratio
- The equilibrium swelling capacity was measured by weighing the sample before and after immersion in distilled water for 24hrs followed by removal of the excess water on the surface with a syringe and filter paper. The swelling ratio is obtained using the following equation:
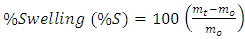 | (3) |
2.3. Characterization
2.3.1. Fourier Transform Infrared (FTIR)
- The Functional groups of the carboxymethylcellulose and superabsorbent hydrogel were investigated using infrared spectroscopy spectrum (Shimadzu IR Tracer -100). Pellets were made from CMC and SAH using KBr. Absorbance levels were measured for wavenumbers of 3800-400 cm-1 respectively.
2.3.2. X-ray Diffraction (XRD)
- The X-ray diffraction patterns of the carboxymethylcellulose and superabsorbent hydrogel were recorded on a D2 PHASER Bruker AXS X-ray diffractometer. The powder samples were dried in a hot air oven at 105°C for 3 h before testing. The scattering angle (2θ) ranged from 10 to 60° at a scan rate of 5°/min.
 | Plate 1. The synthesized superabsorbent hydrogel (SAH) |
3. Results and Discussions
3.1. Cellulose FTIR
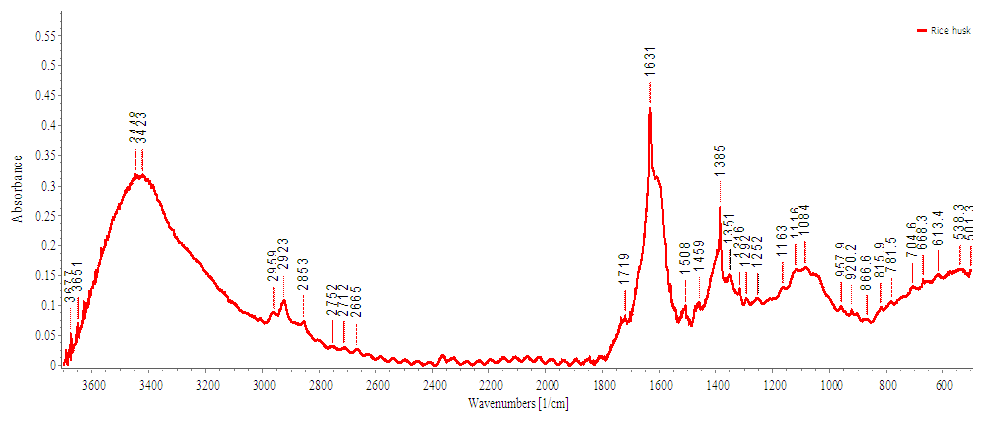
- FTIR spectroscopic investigations gave different absorption bands which are characteristics for cellulose (Figure 3). The broad band at 3432.39 cm-1 is characteristic of the OH-stretching vibration it gives good information about the hydrogen bonds formation, intramolecular and intermolecular hydrogen bond (Wingerson and Richard, 2002). The band at 2924.13 cm-1 represents the C–H stretching vibration. The absorption band at 1631.81 cm-1 is assigned to absorbed water. In addition, the absorption band at 1383 cm-1, represents the symmetric CH2 bending vibration. The hemicellulose and lignin peak at 1508 cm-1 and 1459 cm-1 (which were observed in figure 2) fully disappeared after extraction (figure 3) implying that cellulose has been isolated. The absorption band at 1104.55 cm-1, represents the C–O–C stretching at β-(1-4)-glycosidic linkages (Kondo, 1997).
 | Figure 2. FTIR of rice husk before extraction |
 | Figure 3. FTIR of extracted cellulose from rice husk |
3.2. FTIR of CMC
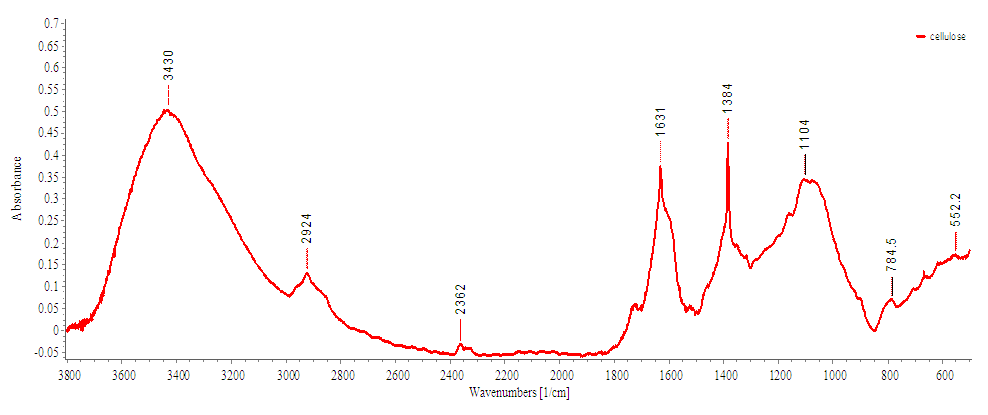
- The IR spectra of the CMC sample prepared to show the typical absorptions of the cellulose backbone as well as the presence of the carboxymethyl group (figure 4). The broad absorption band at 3337 cm-1 is due to the stretching frequency of the –OH group as well as intramolecular and intermolecular hydrogen bonds. The band at 2948 cm-1 is assigned to CH2 stretching vibration (Viera et al., 2007). The new and strong absorption band at 1593 cm-1 confirms the presence of COO- group. The bands around 1459 cm-1 are assigned to –CH2 scissoring and –OH bending vibration (Yeasmin and Mondal, 2015). The band at 1086 cm-1 is due to CH–O–CH2 stretching vibration. The bands at around 650-750 cm-1 is due to the deformation vibration of hydrogen bonds. Based on the spectra, it is believed that the cellulose has undergone carboxymethylation.
 | Figure 4. FTIR of Carboxymethylcellulose |
3.3. Cellulose-Based Hydrogel
- The FTIR Spectrum shown in figure 5 clearly indicates that there was crosslinking between carboxymethylcellulose and 1, 2-ethanediol. The new absorption band at 1730 cm-1 is attributed to the formation ester linkage between carboxymethylcellulose and 1, 2-ethanediol (Coma et al., 2003) thus implying that chemical crosslinking process was successful.
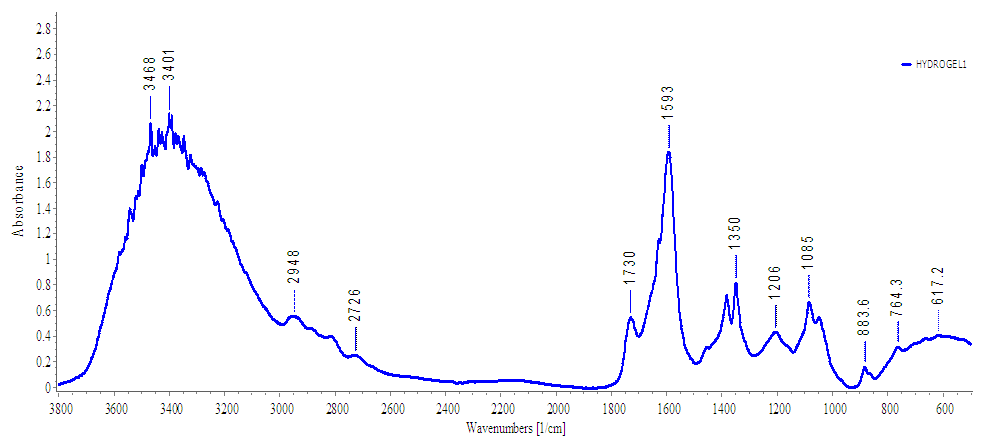 | Figure 5. FTIR of cellulose-based superabsorbent hydrogel |
3.4. X-ray Diffraction
- The hydrogel and CMC were characterized by the X-ray diffraction (XRD) pattern. They show a board peak at 20° associated with the low crystallinity of CMC structure. Both SAH and CMC have a main characteristic peak at 2θ=20.5 and 20 respectively, it is observed that the peak intensity of SAH is higher than the peak of CMC at 20°. It is clear that CMC is not completely amorphous, but have a relatively higher degree of crystallinity. The decrease in crystallinity plays a vital role in hydrogel degradability, water uptake and swelling ratio (Costa-Junior et al., 2009).
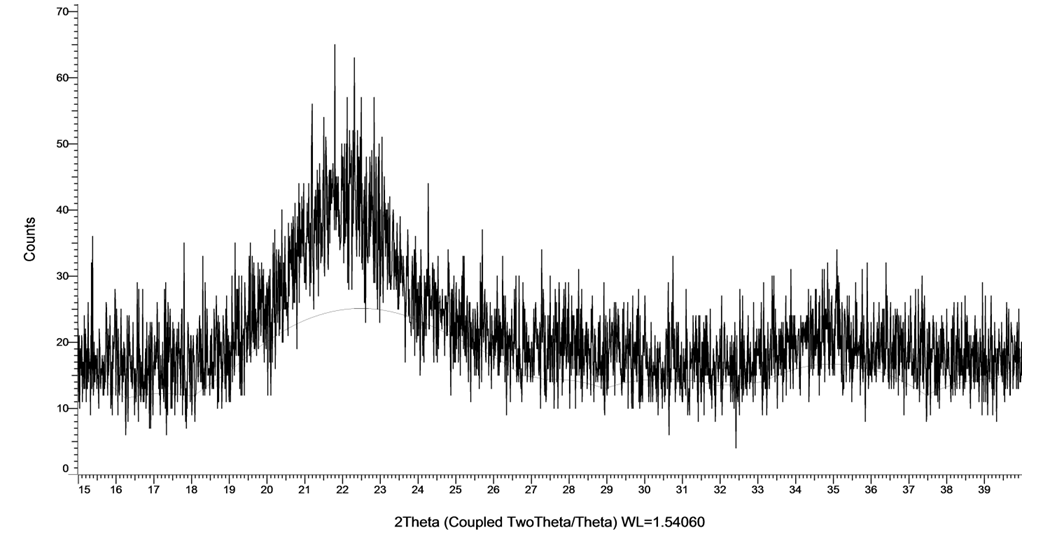 | Figure 6. X-ray diffraction of carboxymethylcellulose |
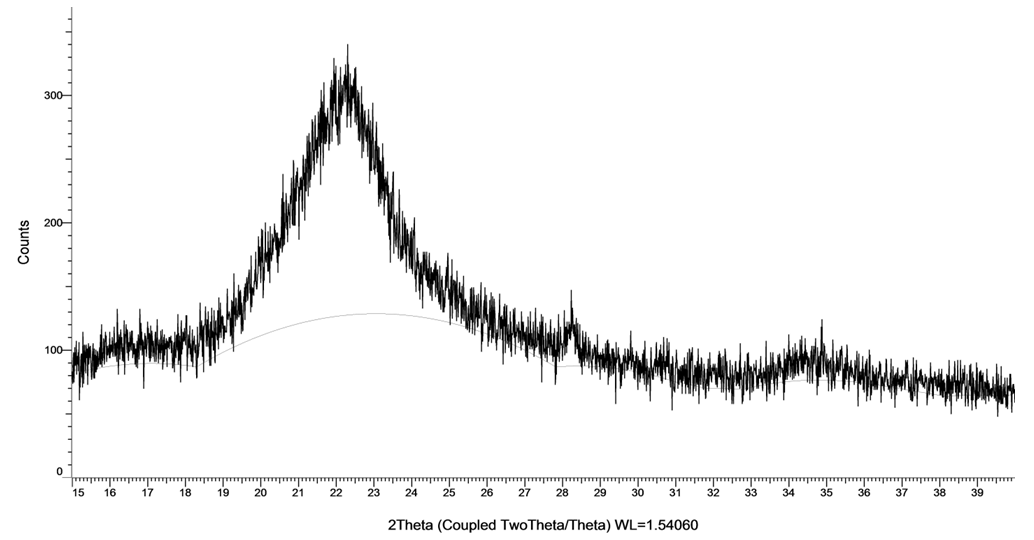 | Figure 7. X-ray diffraction of superabsorbent hydrogel |
3.5. Optimizing Synthesis Conditions for Super-Absorbent Hydrogels
- The Optimum conditions of power output, time, cellulose: linker mass ratio and solvent required for the production of most desirable, stable and high water absorptivity were investigated.As shown in figure 8, the percentage swelling increased with increase in time up to a maximum swelling (1000%) at a time interval of 5 minutes. Thus, on heating at one minute intervals for 5 minutes in the microwave and monitoring the swelling rate of the SAH, it was observed that the amount of water absorbed increased rapidly from 719% in the first minute to 947% in the second minute after which the increment was less gradual to 1000% in the fifth minute. This is because of water penetrating into the hydrogel through capillary and diffusion in the glassy state and then it is absorbed by hydrophilic groups such as carboxylate through the formation of hydrogen bonds. The swelling is driven by repulsion of hydrophilic groups inside the network and osmotic pressure difference between the hydrogel and the external solution. Subsequently, swelling rate gradually slows until the swelling reached up to equilibrium. Similar result was obtained by Rajeev Jindal et al., 2010, “as the time interval is increased, there will no vacant spaces for the water molecule because the porous network of the polymer is saturated”. [This is due to increased cross-linking of hydrogel with time].
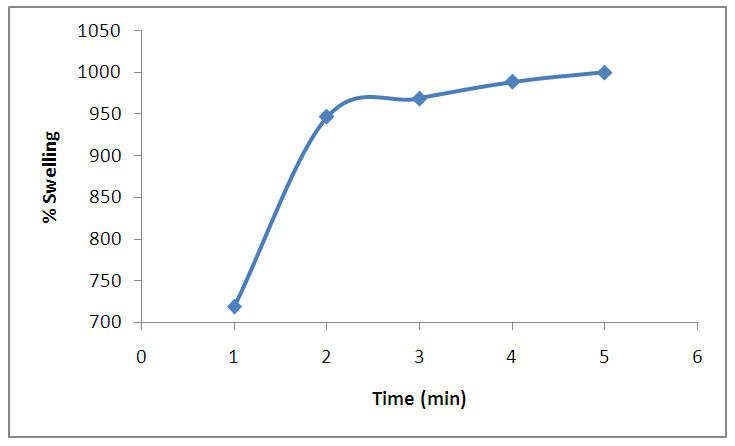 | Figure 8. Effect of time on the percentage of swelling (at Power output 4 equivalent to 280 watts, 10 mL of 1,2-ethanediol, 2 g of CMC) |
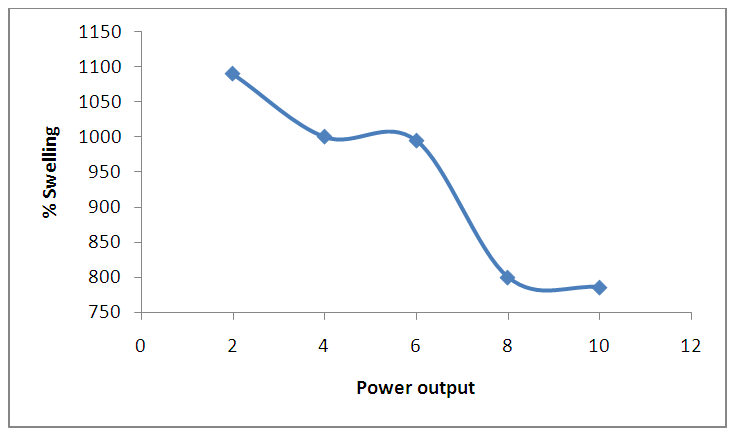 | Figure 9. Effect of power output on percentage of swelling. (at 5 minutes, 10mL of ethanediol, 2 g of CMC) |
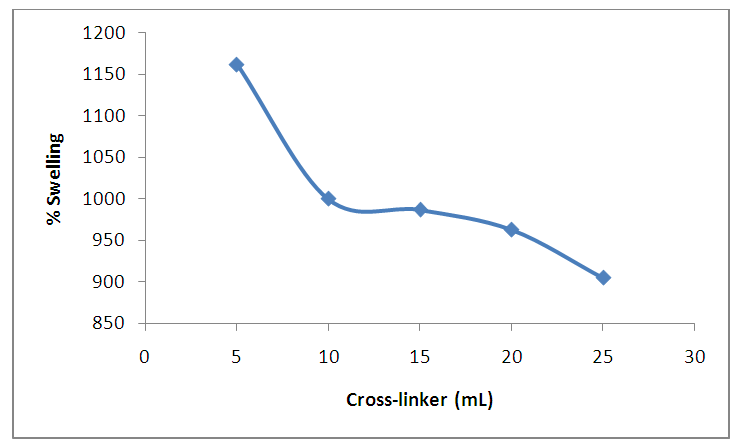 | Figure 10. Effect of amount of cross-linker on the percentage of swelling. (at Power output 4 equivalent to 280 watts, 5 minutes, 2g of CMC) |
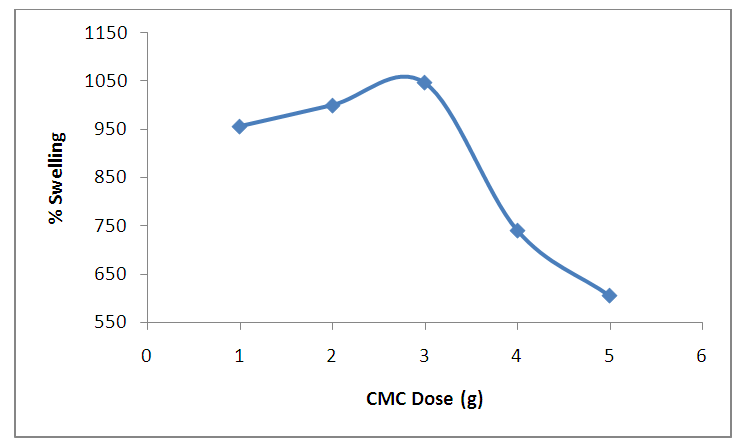 | Figure 11. Effect of CMC dose on percentage swelling. (at Power output 4 equivalent to 280 watts 10 mL of ethanediol, 5 minutes) |
4. Conclusions
- In this work, the superabsorbent hydrogel was successfully synthesized from rice husk cellulose derivatives via carboxymethylcellulose using a microwave, this shows that 1,2-ethanediol can be used as cross-linking agent for the synthesis of SAH as confirmed by using FTIR, XRD. The percentage swelling at (5 minutes, power output of 2, 5 mL of cross-linker (1, 2-ethanediol) and 3 g CMC) gave the optimal of 1162%. The SAH produced from this study using a microwave has advantage of reducing cost, energy, easier and serves as a solution toward using toxic chemicals as crosslinking agents. As part of the recommendation, this SAH can be applied in arid areas for agricultural practices towards addressing drought.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML