-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2019; 9(1): 22-28
doi:10.5923/j.materials.20190901.04

Graphene Oxide to B, N Co-doped Graphene through Tris-dimethylaminoborane Complex by Hydrothermal Implantation
Md. Abdul Mannan1, 2, Yudai Hirano2, Armando T. Quitain2, Michio Koinuma2, Tetsuya Kida2
1Faculty of Science, Department of Chemistry, University of Rajshahi, Bangladesh
2Graduate School of Science and Technology, Kumamoto University, Kumamoto, Japan
Correspondence to: Md. Abdul Mannan, Faculty of Science, Department of Chemistry, University of Rajshahi, Bangladesh.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this present work, we report a simple hydrothermal synthesis of B and N co-doped reduced graphene oxide through trisdimethylaminoborane complex. Maximum B and N atoms with an atomic percentage of 2.30 and 4.12 at.% respectively, were achieved onto the GO framework at the reaction temperature of 250°C. Introduction of B and N into the GO matrix was confirmed by X-ray photoelectron spectroscopy. FT-IR measurement was conducted in order confirm the presence of different functional groups as well as the formation of different bonds such as B–C, C–N, B–O–B etc. XRD and Raman spectroscopy were employed to confirm the defects structures arisen from penetration of boron and nitrogen atoms to the GO lattice.
Keywords: Graphene oxide, Boron nitrogen co-doping, Hydrothermal implantation, X-ray photoelectron spectroscopy, Raman spectroscopy
Cite this paper: Md. Abdul Mannan, Yudai Hirano, Armando T. Quitain, Michio Koinuma, Tetsuya Kida, Graphene Oxide to B, N Co-doped Graphene through Tris-dimethylaminoborane Complex by Hydrothermal Implantation, American Journal of Materials Science, Vol. 9 No. 1, 2019, pp. 22-28. doi: 10.5923/j.materials.20190901.04.
Article Outline
1. Introduction
- Graphene oxide (GO) is a mono-layered material, having some sp2-hybridized carbon together with some sp3-domain, showed unique mechanical, electrical and opto-electronical properties wing to different oxygen functionalities such as hydroxyl, carboxyl, and carbonyl carbons [1–3]. The exclusive property makes GO as a smart material for the invention of variety of nanocomposite materials for the application of high performance microelectronic devices, energy storage materials, and in the biomedical applicable materials [4-6]. The applicability depends on the oxygen functionalities present in GO and might be declined in electrical properties, thermo mechanical stability and carrier mobility of the carbon-based materials [7]. Non-metallic elements such as boron, nitrogen, sulfur, fluorine, phosphorous etc. have been incorporated onto the GO layer to improve the electrochemical properties [8, 9]. Different methods such as chemical vapor deposition, thermal annealing, plasma irradiation etc. have been employed for synthesizing N-doped GO for application of electrical, enhancement of surface and polarization energy [10-14]. B and N co-doped GO for the application of supercapacitor, high performance anode material for Li ion battery and other electromagnetic radiation have been reported [15, 16]. However, drawbacks like incomplete purification and excessive amount of by products were remaining in the resulting GO materials that may cause structural degradation and morphological defects [17].In our previous study, B-doped GO has been reported for the glucose biosensing application [18]. In this research, we have synthesized B, N co-doped GO (BN-r-GO) treating with a mixture of graphene oxide and tris-dimethylaminoborane (TDMAB) complex by simple hydrothermal reaction condition. The BN-r-GO samples were characterized by using different spectrophotometric techniques. The results could be used as a reference for further advanced research.
2. Experimental Methods and Materials
2.1. Chemicals
- The required materials such as sulfuric acid (H2SO4), hydrochloric acid (HCl), sodium nitrate (NaNO3), potassium permanganate (KMnO4), hydrogen peroxide (H2O2), boric acid (H3BO3), glucose, 3,5-dintrosalicylic acid (DNS), sodium potassium tartrate, sodium hydroxide (NaOH), TDMAB etc. all were purchased from Wako Pure Chemical Industries, Ltd., Japan.
2.2. Synthesis of GO
- Graphene oxide (GO) was synthesized by modified Hummer’s method using graphite flake as starting material [19]. In a 1L beaker, 4.0 g of NaNO3 weigh out and concentrated H2SO4 (148 mL) were added and stirred for 30 minutes in an ice bath. Thereafter, KMnO4 (20.0 g) was added slowly to the solution and stirred for 40 minutes at 35°C then, followed by distilled water (184 mL) while mixing for 15 minutes at 95°C, all are done in an oil bath. Further, 400 mL of H2O and about 25 mL of hydrogen peroxide (H2O2) were added and stirred at 4000 rpm for 10 minutes. Then the mixture was washed with 5% HCl and stirred at 4000 rpm for 30 minutes. This action was repeatedly done for three/four times. The next step was exfoliation by sonication for 4∼6 hrs. Then the centrifuged at 10,000 rpm for 30 minutes resulting to the supernatant GO. Finally, it was dried for 3 to 4 days in an oven at 60°C.
2.3. B, N-doping
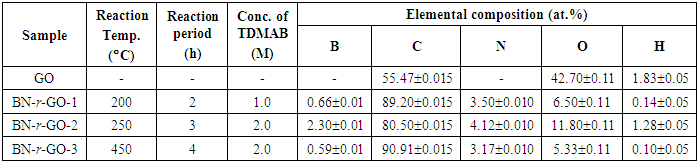
- The as-obtained GO was used for further doping with B and N by simple hydrothermal reaction in which the reactor was equipped with an 8.8 mL inconel batch reactor. A schematic of the synthesis process is shown in figure 1. Typical 0.25 g of GO together with different concentration (1.0 M, and 2.0 M) of the tris-dimethylaminoborane (TDMAB) complex were loaded into the reactor. Thereafter, desired reaction conditions such as temperature, pressure and time were set. The reaction scenarios are shown in table 1. The resulting samples were referred to B, N co-doped reduced graphene oxide (BN-r-GO) and were collected, washed, filtered and dried overnight in an oven at 60°C. Finally, the samples were characterized by using different instrumental techniques such as FT-IR, XRD, Raman, XPS etc.
 | Figure 1. Schematic of the synthesis process of graphene oxide, doping of boron and nitrogen by hydrothermal reaction using TDMAB as the precursor |
|
2.4. Characterization
- FTIR spectroscopy (FTIR-4100, JASCO) was used to determine different functional groups that were accumulated in GO and BN-r-GO samples. The measurements were carried out in the wavelength range of 4000 to 400 cm–1. X-ray photoelectron spectroscopy (Perkin Elmer Phi 1600 ESCA) was used to determine the elemental compositions. The XPS was performed at Kumamoto University Instrumental Centre, Japan in which the AlKα was used as the X-ray source for irradiation of the sample surface. Crystallographic structure was determined by X-ray diffractometer (Rigaku, MiniFlex600) using CuKα radiation source. Diffraction data were taken at 2Θ= 5 to 90° with step energy 0.02 eV. Raman spectroscopy was used to evaluate the microstructure of the synthesized GO samples using JASCO NRS-3100 Laser Raman spectrophotometer. The Raman shift was recorded at 500–4000 cm–1 wavelength region.
3. Results and Discussion
3.1. FT-IR Analyses
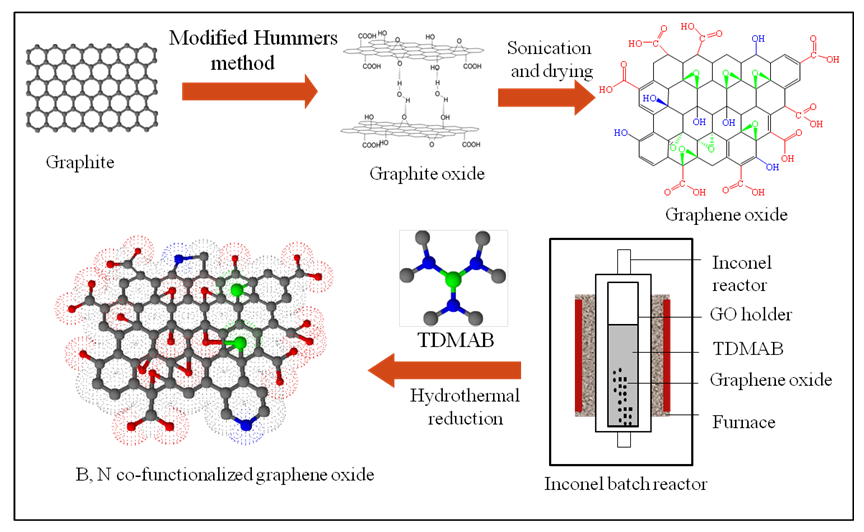
- The functional groups created in the as-synthesized GO and BN-r-GO were assessed by FT-IR spectroscopic measurement and the spectra are shown in figure 2. Five peaks are seen in the spectrum which are the characteristics of GO. The peaks at ∼3300 cm–1, 1736, 1620, 1232, and 1060 cm–1, are assigned for GO by the stretching vibration of hydroxyl (–OH), carbonyl (C–O), aromatic (C=C), epoxy (C–O–C) and alkoxy (C–O) bonds, respectively, [20, 21]. In the spectra of BN-r-GO, the hydroxyl peak became sharp and observed at around 3440 cm–1 due to the reduction of GO. There are six additional new peaks observed at 3740 cm–1, 1643, 1543, 1200, 800 and 659 cm–1, respectively. These new peaks in the spectra of BN-r-GO could be assigned for the stretching vibration of N–H, C=C, C–N, B–C, -C–O–C-, O–B–O bonds, respectively [22-25]. From the spectral analyses, therefore, we could suggest that the –N–H, C–N, O–B–O and B–C bonds have been created in the GO network after boron and nitrogen doping.
 | Figure 2. FT-IR spectra of as-synthesized GO and BN-r-GO samples |
3.2. XRD Analyses
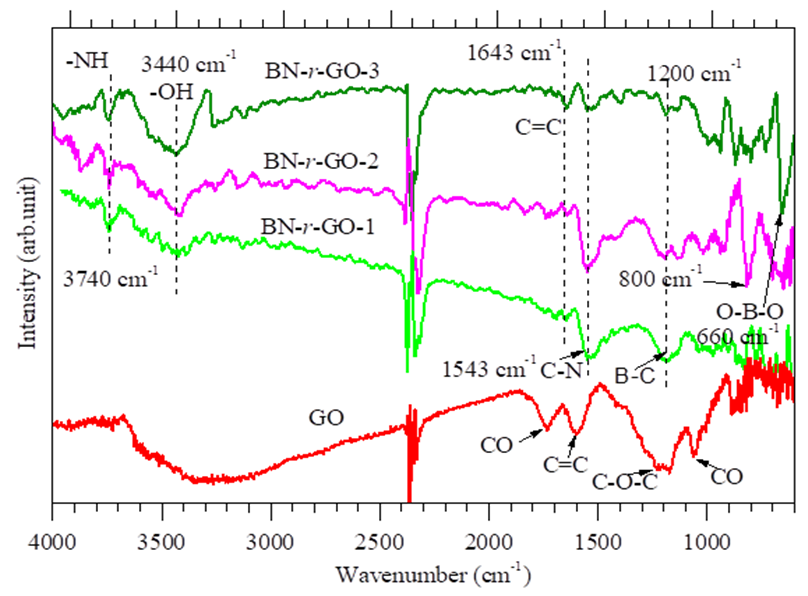
- Crystalline structures of GO and BN-r-GO were investigated by XRD analyses and the patterns are shown in figure 3. A strong and intense peak at around 2Θ=10 (d=0.44 nm) has been observed which is assigned for the non-functionalized crystalline GO [26, 27]. This characteristics peaks of GO in the XRD pattern is completely disappeared in those of the BN-r-GO. This indicated that the oxygen containing groups of GO were efficiently removed at certain percentage. Meanwhile, a broad peak at around 2Θ=25° (d=0.17∼0.18 nm) is seen after hydrothermal reaction with TDMAB. This new peak (2Θ=25°) suggests simultaneous exfoliation and reduction of the GO with boron and nitrogen atoms [28]. A significant decrease in the interlayer spacing distance from d=0.44 to d=0.17∼0.19 nm also support incorporation of B and N atoms into the GO layer. The absence of the peaks associated with oxygen functionalities in the FT-IR spectra agrees well with the XRD results. This findings has also been coincided with the XPS analyses, since, the atomic concentration of B and N were found to be 2.30∼0.59 and 3.17∼4.12 at.%, respectively (table 1) expressed in the following section.
 | Figure 3. XRD patterns of the as-synthesized GO and BN-r-GO samples |
3.3. XPS Analyses
- X-ray photoelectron spectroscopy (XPS) measurements have been carried out in order to further confirmation of element composition and chemical environment in the region of boron, carbon, nitrogen and oxygen atoms in BN-r-GO samples. The XPS survey scan spectra showed the evidence of B, C, N and O elements and are shown in figure 4. The elemental compositions (at. %) were calculated from the XPS peak intensities and are tabulated in table 1. It is seen that B and N incorporation is increased up to 2.30 and 4.12 at.%, respectively with the increase of the concentration of TDMAB complex. High resolution core shell XPS spectra of B1s, C1s, N1s and O1s are carried out in order to explain the chemical environment around the B, C, N and O atoms of the BN-r-GO. The core shell spectra and the deconvoluted peaks of B1s, C1s, N1s and O1s for the typical sample BN-r-GO are shown in figure 5.
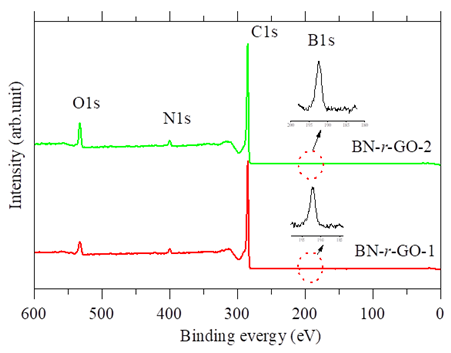 | Figure 4. Survey scan XPS spectra of BN-r-GO samples |
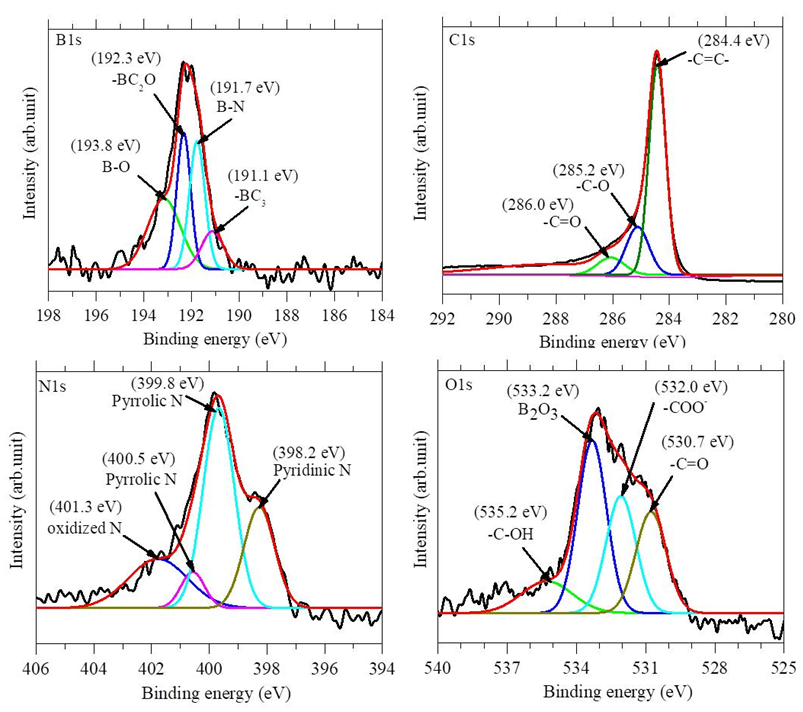 | Figure 5. Deconvoluted high resolution core shell B1s, C1s, N1s and O1s XPS spectra for a typical BN-r-GO sample. The spectra were taken at the step energy of 0.02 eV |
3.4. Raman Analyses
- Raman spectroscopy was used for indentifying the crystalline structure and quality of the as-received GO as well as the BN-r-GO. The measurements have been done in the spectral region of 1000–2000 cm–1 using same laser power (632 nm laser excitation) for all the samples. The Raman spectra of the typical samples are shown in figure 6. It is seen that the D band intensity is increased while the G band intensity is decreased for the as-synthesized GO and the BN-r-GO in comparison to that of the pristine graphite flake. The increment of intensity and broadening of both the D and G band confirmed the assimilation of oxygen containing functional groups in the GO framework. The noteworthy increment of D and decrease of G band in BN-r-GO also confirmed the assimilation of B and N elements in the GO matrix. This functionality is caused by the loss of crystalline structure of the GO as well as the BN-r-GO. Forward shifting of the G band position from 1592 to 1598 cm–1 of BN-r-GO suggested inclusion of B and N atoms in the GO lattice leading to increase in disordered structure [39]. The relative intensity ratio ID/IG is clearly greater than unity which is demonstrated the increase in the defect upon doping the hetero-atoms such as B and N that might leads to the considerable changes in the structural properties, electrical and other physicochemical properties [40].
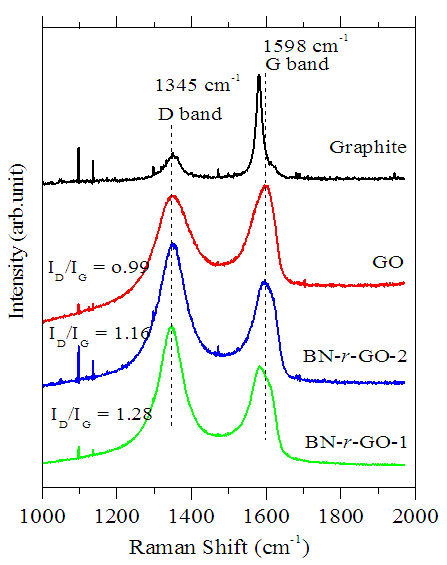 | Figure 6. Raman spectra of pristine graphite (top spectrum), as-received GO, and BN-r-GO samples |
4. Conclusions
- Boron and nitrogen were successfully co-doped into graphene matrix by simple hydrothermal reaction using tris-dimethylaminoborane complex as boron and nitrogen source. This method gives upto 2.3 at.% of boron and 4.12 at.% of nitrogen doping onto the GO lattice which were confirmed by XPS analyses. The FT-IR analyses confirmed the presence of different functional groups and the B–C, C–N as well as B–O–B bonds in the BN-r-GO matrix. The defects crystalline structural arisen from the B and N introduction into the GO lattice and that was confirmed by XRD and Raman spectroscopic analyses. The as-received BN-r-GO could be recommended as different electro analytical applications such as biosensor, capacitor, electrochemical catalyst and in fuel cells etc.
ACKNOWLEDGEMENTS
- The author gratefully acknowledged The President of JASSO, Japan, for providing financial support under JASSO Fellowship Program.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML