-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2018; 8(4): 73-78
doi:10.5923/j.materials.20180804.02

Investigation of Methyl Orange Adsorption from Water Using Acid Activated Makoro Clay
Misael Silas Nadiye-Tabbiruka, Lebogang Lungani, Segametsi Chaloba, Wilfred Ddamba
Department of Chemistry, University of Botswana, Botswana
Correspondence to: Misael Silas Nadiye-Tabbiruka, Department of Chemistry, University of Botswana, Botswana.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The removal of Methyl Orange (MO) from its solution in water by Hydrochloric acid (HCl) activated Makoro clay was investigated by studying the kinetics and thermodynamics of the MO adsorption onto the clay. The kinetics was found to follow pseudo second order mechanism indicating either a highly unlikely third order reaction, or two simultaneous second order processes, thought to be adsorption onto the external and internal surfaces of the clay associated to the two observed equilibria. The thermodynamic data fitted Freundlich model best indicating surface heterogeneity. The low change in heats and entropy of adsorption indicate physisorption. The acid activated clay was found to be an initially fast and efficient remover of methyl orange from water, which later slowing down during the second equilibrium.
Keywords: Adsorption from Solution, Water Purification, Acid Activated Clay
Cite this paper: Misael Silas Nadiye-Tabbiruka, Lebogang Lungani, Segametsi Chaloba, Wilfred Ddamba, Investigation of Methyl Orange Adsorption from Water Using Acid Activated Makoro Clay, American Journal of Materials Science, Vol. 8 No. 4, 2018, pp. 73-78. doi: 10.5923/j.materials.20180804.02.
Article Outline
1. Introduction
- The increase in demand of better, fresher and healthier water in our community today, has led to a quest of finding more ways to purify water. Investigations to find natural and more user friendly methods are in progress. Dyes especially azo dyes are amongst the most common contaminants in water and are toxic to the environment and human lives [1]. Azo dyes do not degrade easily due to their complex structures which consist of one or more benzene rings. Therefore, removal of dyes is an important aspect of wastewater treatment before discharge into the environment [2].Many separation techniques such as adsorption [3], photocatalysis [4], oxidation [5], and nanofiltration [6] have been applied in removal of dyes from solutions. Adsorption is considered as a major industrial separation technique for the purification of effluent media due to its low cost, high efficiency and easy operation [7]. Activated carbon and clay are among many different adsorbents used in the adsorption technique [8]. Activated carbon has been successfully used in removing coloured organic species and is the most widely used adsorbent due to its high capacity of adsorption of organic materials [8, 9] and its effectiveness and versatility [10, 11]. However, it is limited as it has slow adsorption kinetics and low adsorption capacity of bulky adsorbate because of its microporous nature, disposal problems and also the high cost and difficulty of regeneration as such, a search for a more effective adsorbents such as clay derivatives has becomes important [12-14]. Clays have high specific surface area due to presence of nanopores hence they fall under the category of the most effective adsorbents [15]. In the past natural clays have always been used to purify water to certain degrees of purity, but to explore this technique further clays have been activated to increase porosity and hence increase surface area of activity in order to enhance their adsorption ability. The two most commonly used methods of activating clays are the physical and chemical methods. Physical methods include use of steam [16] whilst the chemical methods include acid and alkaline activation [17]. Acid activated clay result in improved efficiency (120%) compared with alkali activated clay. The acids most widely used for acid activation include hydrochloric (HCl) and sulfuric (H2SO4) acids because they increase specific surface area, due to increased porosity which results in increased adsorption capacity of the activated clay [17].In this study methyl orange was used as water polluting dye and HCl activated Makoro clay was used as the adsorbent. According to a study by Ekosse [18] Makoro clay exist in different colours, light gray to reddish brown, yellowish brown to dark brown colour and light gray, its particle size ranges between 2 and 10 µm and has a higher surface area. The clay also has well developed kaolinite crystals and relative porous aggregates.Methyl orange is a carcinogenic synthetic azo dye which is widely used in textile industries, in paper printing, and in research laboratories [19, 20]. It causes allergies, and hyper sensitivity when consumed and on long term skin contact. It is also metabolized to aromatic amines by intestinal microorganisms. Because it contains nitrogen atoms in its structure, the reductive amides in the liver also catalyze the reductive cleavage of azo linkages to produce aromatic amines which can lead to cancer [21]. The chemical structure of this dye is given in figure 1. Methyl orange is stable, shows low biodegradability and is soluble in water hence it is difficult to remove from aqueous solutions by common water purification/treatment methods.
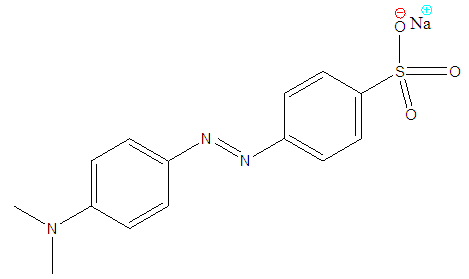 | Figure 1. Chemical structure of methyl orange (Sodium 4-{(4-dimethylamino) phenyldiazenyl}benzenesulfonate) |
2. Experimental
2.1. Materials and Methods
2.1.1. Makoro Clay
- Samples of clay were collected from various parts of the deposit at Makoro clay mine in Makoro village near Palapye in Botswana. The clay was mixed with excess deionized water (5L/kG) and stirred for 48 hours at 200rpm. It was then allowed to settle for 24 hours. The top water suspension which is composed of fine clay particles was decanted and filtered under vacuum. The clay was ground with mortar and pestle and was heated (calcined) for 3hours at 413.15K.The clay (50gm) was activated by leaching with 200mL of 4M HCl acid for 2 hours in a sonicator at 363K. Leaching also removed iron ions (Fe+2, Fe+3) from the clay, as their salts absorbed at a similar wavelength as the dye used for the study (MO). The acid was filtered off, neutralized with sodium hydroxide (NaOH) to minimize acid pollution. Titration of a sample of the initial acid filtrate showed that the acid concentration was lower than the initial solution indicating a reaction with the clay occurred. The clay was washed with excess deionized water (2L/100g) to remove iron salts and excess acid until there was no precipitation of iron hydroxide on addition of sodium hydroxide. The clay was dried in an oven at 383.15K reground and then sieved using a 500 µm sieve.
2.1.2. Methyl Orange
- A predetermined optimum concentration of 0.001M of MO stock solution, to give manageable kinetics study, was prepared by dissolving 0.082g of the dye in deionized water. The solution was transferred into a 250ml volumetric flask and was filled to the mark using deionized water. The stock solution was used to make six standards of molar concentrations of 2x10-5, 4x10-5, 6x10-5, 8x10-5, 10x10-5 and 12x10-5 M by serial dilution.
2.2. Experimental Procedures
2.2.1. Kinetic Studies
- In each experiment, an accurately weighed 4g of acid activated clay was placed in a 250 ml reaction bottle containing 200 ml of methyl orange. The mixture was agitated in a shaking water-bath for a given period of time at a speed of 120 rpm at constant temperature (298 K). Five 5 ml of the mixture were collected at various predetermined time intervals for a period of 3 hours or longer, was centrifuged and filtered using Whatman filter papers. The absorbance of the residual methyl orange was obtained using a UV/VIS spectrophotometer at a wavelength of 465 nm. These were then used to obtain the concentration from a calibration curve. The amount of the dye adsorbed by the clay was obtained from these concentrations using the following equation;
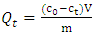 | (1) |
2.2.2. Thermodynamic a Studies
- A batch method was used for obtaining adsorption isotherms, 1 g of acid activated clay was added to each of six 100 ml reaction bottles containing 50 ml of different initial concentrations of methyl orange (2 x 10-5 M, 4 x 10-5 M, 6 x 10-5 M, 8 X 10-5 M and 10 x 10-5 and 12x10-5). The mixtures were agitated in water bath shaking at a speed of 120 rpm for at least 3 hours at a constant temperature of 298 K. the equilibrium concentrations of the dye were analyzed using a uv/vis spectrophotometer as described in kinetics experiments. These experiments were repeated three times or more to ensure reproducibility. The amount of dye adsorbed at equilibrium was calculated using the equation below;
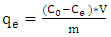 | (2) |
3. Results and Discussion
3.1. Kinetics Study
3.1.1. Effects of Contact Time and Concentration on Kinetics
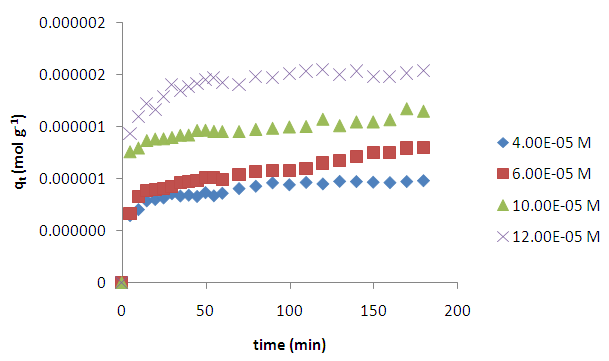 | Figure 2. Effects of time and varying initial concentration on the adsorption of methyl orange onto acid activated clay at 298 K |
3.1.2. Effects of pH
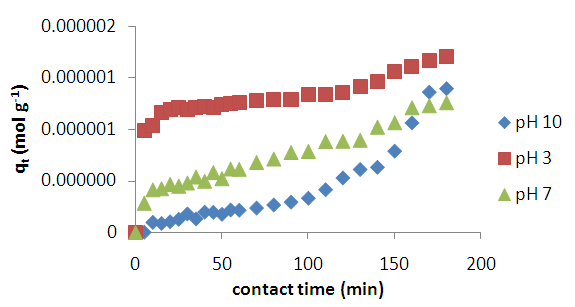 | Figure 3. pH Effects on adsorption of methyl orange onto acid activated clay particles at 298 K |
3.1.3. Effect of Temperature
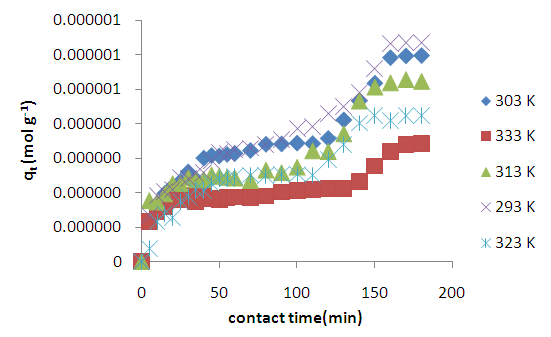 | Figure 4. Effects of temperature on the adsorption of Methyl orange onto acid activated clay particles |
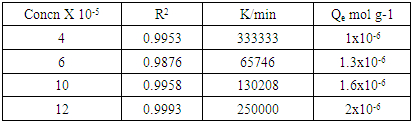
3.1.4. Analysis of Adsorption Kinetics Results
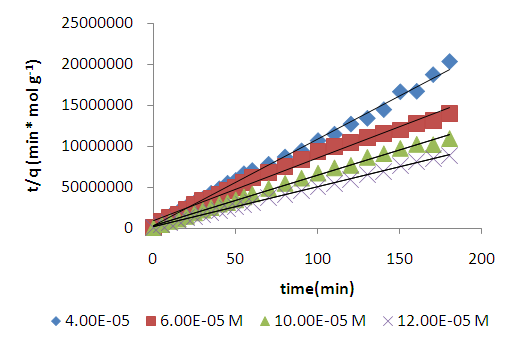 | Figure 5. Lagergren pseudo second order model plot for methyl orange adsorption onto acid activated clay at 298 K |
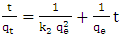 | (3) |
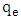 is the amount adsorbed at equilibrium,
is the amount adsorbed at equilibrium, 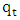 is the amount adsorbed at time t.
is the amount adsorbed at time t.
|
3.2. Thermodynamics Study
3.2.1. Langmuir Adsorption Isotherm
- It is not surprising that the data did not fit Langmuir isotherm. It is very unlikely that acid activated clay would have uniform adsorption sites even in the nano state. The pores resulting from activation will provide a variety of adsorption environments thus resulting in heterogeneity.
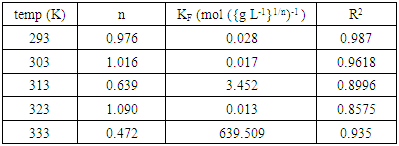
3.2.2. Freundlich Adsorption Isotherm
- The linearized form of the Freundlich isotherm is given below;
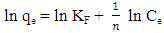 | (4) |
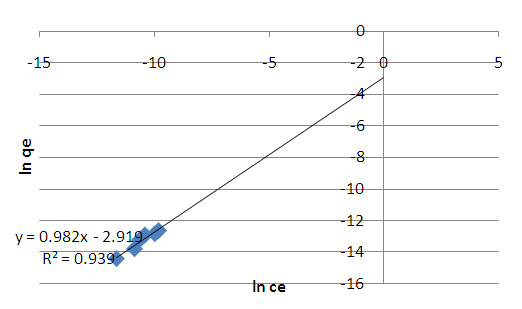 | Figure 6. Freundlich isotherm for adsorption of methyl orange onto acid activated clay at 298 K |
3.2.3. Thermodynamic Parameters
- Gibb’s free energy change (∆G), enthalpy change (∆H), and entropy change (∆S), were obtained so as to confirm the characteristics of the adsorption process. The parameters were obtained using the following equations [27];
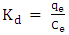 | (5) |
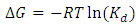 | (6) |
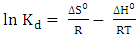 | (7) |
4. Conclusions
- Acid activated Makoro clay samples were successfully prepared by acid leaching, grinding and sonication. Kinetics data showed that adsorption follows the Pseudo second order better than the pseudo first order mechanism. This would indicate a third order reaction. However, such a mechanism is very rare hence it is suggested that adsorption takes place on two different environments just as was found with coal in earlier work [25] that is the clay particle external surface and the internal surface in the pores created by acid activation. Consequently two simultaneous second order processes, known to display pseudo second order kinetics as well, are in operation in this case. Adsorption capacity of the activated clay increases from 1x10-6 moles/g at an initial concentration of 4x10-5M to 2x10-6 moles/g at 12x10-5M. The adsorption enthalpy and entropy change were found to be -27.37kJmol-1 -0.106 kJ/K indicating physical adsorption. The thermodynamics data fitted Freundlich isotherm best indicating heterogeneity. Thermodynamics studies at various temperatures showed the fitting to improve with decreasing temperatures as shown by the regression analysis (table 2). This confirms that physisorption is in operation.
|
ACKNOWLEDGEMENTS
- The authors wish to acknowledge support from the Department of Chemistry technical staff for help with instrumentation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML