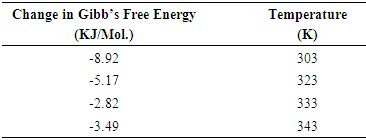-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2018; 8(3): 58-63
doi:10.5923/j.materials.20180803.03

Corrosion Inhibition and Adsorption Mechanism of Mild Steel by Alchornea Cordifolia Leaf Extract in Sulphuric Acid Solution
E. Oparaku 1, E. Osarolube 2, W. O. Aka 1
1Department of Physics and Astronomy, University of Nigeria, Nsukka, Enugu State, Nigeria
2Department of Physics, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria
Correspondence to: E. Oparaku , Department of Physics and Astronomy, University of Nigeria, Nsukka, Enugu State, Nigeria.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The corrosion inhibition of mild steel in 0.2M Sulphuric acid solution at room temperature by Alchornea Cordifolia leaf extract was studied using weight loss technique. The concentration of the extract varied from 0.1g/L through 0.5g/L, and their effects were closely investigated on the corrosion rate of mild steel. The corrosion penetration rates were compared for both in the absence and in the presence of the leaf extract at different concentration for 21 days. The experimental results showed Alchornea Cordifolia leaf extract to be an efficient inhibitor in the acidic medium with general decrease in the corrosion rate as the concentration of the extract increases. Furthermore, it was also observed that at higher temperatures, there were enhanced inhibitor efficiencies for individual concentrations. For example, at 60°C, 0.4g/L inhibitor concentration yielded an inhibitor efficiency of 80.68%, while at 70°C, 0.4g/L resulted to inhibitor efficiency of 82.78%. This was observed generally on average. At elevated temperatures of 50°C, 60°C, and 70°C for 2 hours, the corrosion rates were less in media containing the leaf extract than those without the leaf extract. Hence, the Alchornea leaf extract was more effective at elevated temperatures. The adsorption of the inhibitor on the mild steel obeys the Langmuir adsorption isotherm adsorption. This suggests physical adsorption as the inhibitor mechanism. Also, the negative nature of the change in Gibb’s free energy showed that the adsorption was spontaneous.
Keywords: Corrosion, Mild Steel, Alchornea Cordifolia, Suphuric acid
Cite this paper: E. Oparaku , E. Osarolube , W. O. Aka , Corrosion Inhibition and Adsorption Mechanism of Mild Steel by Alchornea Cordifolia Leaf Extract in Sulphuric Acid Solution, American Journal of Materials Science, Vol. 8 No. 3, 2018, pp. 58-63. doi: 10.5923/j.materials.20180803.03.
Article Outline
1. Introduction
- In practical terms, the usefulness of engineering materials is affected by the interaction with the environment in many ways. Material degradation is the outcome of this condition. Therefore corrosion is the wearing away of materials due to chemical reactions. Apart from metals that primarily suffer electrochemical attack, corrosion can also occur in polymers and ceramics. The other materials can only be deteriorated through attack from chemical directly.In industries such as pulp and paper industry, power generation, underground structures, chemical and oil industries, metals are used in over 90% of construction process [1].Metals are usually exposed to the action of bases or acids in the industries. Processes in which acids play a very important role are acid pickling, industrial acid cleaning, cleaning of oil refinery equipment, oil well acidizing and acid descaling [2]. The exposures can be severe to the properties of the metals and thus lead to sudden failure of materials in service. There is therefore the need to study the corrosion behaviour of metals when exposed to various environments, as this is an important factor in material selection that determines the service life of the material. Mild steel and high carbon steels are classified as ferrous metals (they contain a large percentage of iron). Carbon steels are essentially iron-carbon alloys. They are sometimes subdivided by the broad range of carbon content, which include: (a) mild or low carbon steel (0.08 – 0.30% carbon) (b) medium carbon steel (0.3 – 0.5% carbon) and (c) high carbon steel (0.55 – 1.40 carbon). For many years, mild steel plates and rod-sections have been used as structural members in bridges, buildings, pipelines, heavy vehicles, in welded plate form for the construction of ships storage vessels and numerous other applications [3, 4]. High carbon steel (having a higher carbon content than mild steel) is harder and stronger, and yet least ductile of all the carbon steels. It is mainly used for the manufacture of metal cutting tools like hammers, saws, forging die blocks, axes, knives, drills and wood [5].Threats to environment come from different sources. Corrosion of pipelines, equipment, and other structures is the main contributors of such threats. In 1993, the bridge failure in Mianus was due to corrosion, it was internal rusting of the bearing that resulted in misalignment of the road slab beyond its support. The load of three vehicles on the bridge at the same time resulted in failure and collapse of the slab and the three divers died. When these structures made of these metals and their alloys are improperly maintained, they degraded or deteriorate gradually by action and presence of some elements and compounds in the atmosphere. This process whereby metals and their alloy undergo gradual degradation or deterioration by action and presence of substances in the environment is called corrosion. Hence, corrosion can be defined as gradual destruction and deterioration of poorly maintained metallic substances in the environment; corrosion is an electrochemical process and starts at the surface of the metal [6].Sulphuric acid is widely used in industries such as pickling, cleaning and descaling, industrial cleaning agent, production of chemicals, etc. Mild steel is used in these environments due to its easy availability, low cost and excellent physical properties, but its use and lifespan is restricted in these conditions due to its susceptibility towards corrosion. To complement its low cost and economic value, the most effective means of addressing the corrosion of mild steel is with the use of inhibitors, especially organic compounds. Though many compounds show good anticorrosive activity, most of them are highly toxic to both human beings and the environment. These toxic effects and ecological problems associated with the discharge of such materials have resulted in the development of other efficient and environmentally acceptable inhibitors. Hence, the recent trend is the search for environmentally friendly chemicals. Most natural products are nontoxic, bio-degradable and readily available in abundance for use in aggressive solutions in contact with the steel surface in order to inhibit the corrosion reaction and significantly influence the kinetics of the electrolytic process. A number of organic compounds [7-14] are known to be applicable as corrosion inhibitors for steel in acidic environments. Such compounds typically contain nitrogen, oxygen or sulphur in a conjugated system, and function via adsorption of the molecules on the metal surface, creating a barrier to corrosive attack [15]. In frequent instances the addition of small quantities of special chemicals to large volumes of corrosive solutions can be quite effective in reducing corrosion. Such chemicals are called corrosion inhibitors. At the simplest level a corrosion inhibitor is a substance which when added in small concentration to an environment effectively reduces the corrosion rate of a metal exposed to that environment. Because of the toxic nature and high cost of some chemicals used like chromate and nitrate, it is necessary to develop environmentally acceptable and less expensive inhibitors [16]. The selection of inhibitor is controlled by its economic availability, its efficiency to inhibit the substrate material and its environmental side effects. Most of the excellent acid inhibitors for corrosion of steel in acidic medium are organic compounds containing nitrogen, oxygen and/or sulphur atoms [17-20]. The inhibiting action of these compounds is attributed as a first stage, to the adsorption of the additives to the metal/solution interface. The adsorption process depends upon the nature and surface charge of the metal, the type of aggressive media, the structure of the inhibitor and the nature of its interaction with the metal surface. Metallic material constitutes a great part of construction material elements in industries, agricultural equipment, oil and gas and petrochemical, medical services, process and allied industries. In these industries, the metallic material as a result of interaction with its environment loses its integrity over a period of time [21]. As such, the material cannot perform the intended function effectively and reliably. Some of these environments are atmosphere, aqueous solution, solids, acids and bases, inorganic solvents, molten salts, liquid metals, human body etcetera. At times, the effect of the loss in integrity may be very severe as to result in loss of valuable production time, accident and in the extreme death. The cost associated with the problem is enormous and its influence in the economy of a nation is significant. It has been estimated that approximately 5% of industrialized nations’ income is spent on corrosion incidental problems. For instance, the cost of the problem to the U.S. economy is put at $297 billion annually [22].We can prevent corrosion by using metals that has the capacity naturally protect itself from corrosion through formation of film layers; however, such metallic alloys are either very exorbitant or poisonous. The toxicity of commercial inhibitors plus the rising strictness of the environmental standards has boosted intensive research into the existing or novel substances as potential inhibitors of corrosion. These factors have shifted the focus of study to non-toxic “green” inhibitors of corrosion. Green inhibitors include substances that protect metals from corrosion without having any negative effect on the environments and life forms. This also leads to the idea to investigate the possibilities of using tinctures and extracts of medicinal herbs as potential inhibitors.According to the estimations of the world experts in this field, corrosion of iron and its alloys in the world during a period of one year “eats” over thirty percent annual world production of iron.Also, in our environment, corrosion of metals, especially mild steel, has resulted in many mechanical failures of structures around us. It implies that corrosion will likely impact on its mechanical features, hardness, and structure of mild steel internally [23].Alchornea Cordifolia belongs to the family of Euphorbiaceae, having a chromosome number of 36.
 | Figure 1. Alchornea Cordifolia Leaves |
2. Experimental Details
2.1. Work Materials and Specimen Preparation
- The test coupons were dimensioned 30mm by 30mm using a trisquare and a scriber. They were cut and shaped using guillotine machine and hand respectively. A 2mm diameter hole was drilled on the test coupons to allow polystyrene thread to pass through. This is for easy handling of the coupons during the experiments. The coupons were weighed to obtain their initial masses which were used to calculate their densities and also weight loss after corrosion or inhibition using the dimensions of the coupons.
2.2. Preparation of Acid and Inhibitor Extract
- The corrodent used was Tetraoxosulphate VI acid solution (H2SO4).The corrosion penetration rate of the acidic medium on the mild steel is calculated as follows:
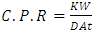 | (1) |
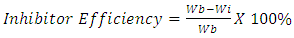 | (2) |
2.3. Experimental Procedure
2.3.1. Weight Loss Technique at Room Temperature
- This is inhibited and non-inhibited setup using 0.2M Sulphuric acid on coupons of Mild Steel. The masses of the coupons were measured using the electronic balance. Two coupons were immersed in six beakers each containing the corrodent with and without inhibitor concentrations: 0mg/L, 0.1mg/L, 0.2mg/L, 0.3mg/L, 0.4mg/L, and 0.5mg/L. after each 24hours, the coupons in each beaker were removed. The volume of the medium for each beaker was 400mL, which comprised of both acid and the inhibitor for the inhibited medium and only the acid for non-inhibited medium. The set up was done in such a way that the two coupons in each beaker were removed, cleaned, dried, and weighed for every 24hours. This lasted for 7days. Similarly, after 14days, 12coupons, 2 from each beaker were also removed, cleaned, dried, and weighed. The same was also done after 21days. The essence of two coupons for each medium was to deduce the average weight loss.
2.3.2. Weight Loss Technique at Elevated Temperature
- In this setup, 3 sets of coupons of mild steel were immersed two each in six beakers containing the corrodent with inhibitor concentrations: 0mg/L, 0.1mg/L, 0.2mg/L, 0.3mg/L, 0.4mg/L and 0.5mg/L. each set of the beakers were heated at elevated temperatures of 50°C 60°C, and 70°C, with the same inhibitor concentration for about 2hours. After each removal, the coupons were cleaned, dried, and weighed.
3. Results and Discussion
- The tables below show the results obtained using the weight loss technique for inhibited and non-inhibited at room and elevated temperatures.
3.1. Data Analysis and Discussion of Findings
3.1.1. Results of Weight Loss Technique at Room Temperature
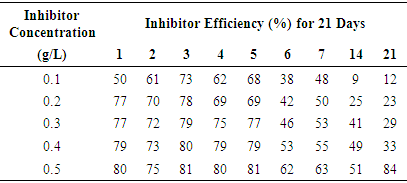
- For the non-inhibited (control) samples, it was noticed that the corrosion penetration rate increased with exposure time. For the inhibited samples, it was also observed in table 1 that as the concentration of inhibitor was increased, the inhibitor efficiency also increased. The same was also observed for the rate of corrosion which decreased as concentration of inhibitor was increased. This was consistently observed for the first seven days, and then the fourteenth and twenty first days respectively. The plot of inhibitor efficiency versus inhibitor concentration shows that as the inhibitor concentration of the medium increases, the efficiency of inhibitor increases as well.From the results obtained at normal room temperature, Alchornea Cordifolia leaf extract as an organic inhibitor of mild steel drastically hinders or minimizes its corrosion by inhibiting it from the attack by the sulphuric medium. This was drawn from the high inhibitor efficiencies of 79.38%, 80%, 81.24%, and 84.35% recorded.
|
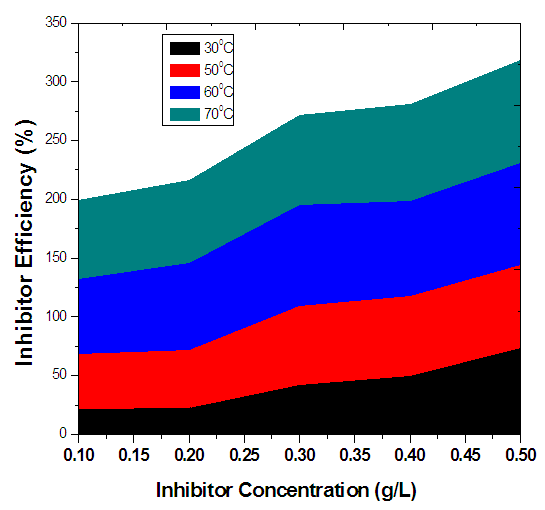 | Figure 2. Variation of Inhibitor Efficiency with Inhibitor Concentration of Alchornea Cordifolia leaf extract for mild steal in 0.2M Sulphuric acid at 30°C, 50°C, 60°C, and 70°C for 2Hours |
3.1.2. Results of Weight Loss Technique at Elevated Temperature
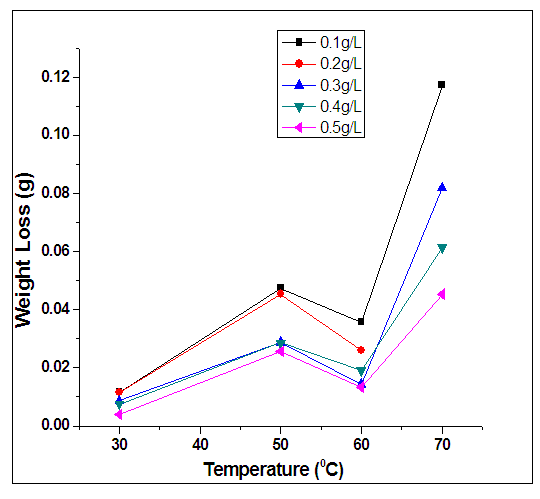
- At elevated temperatures, rise in concentration of inhibitor resulted to reduction of both weight loss and rate of corrosion. Conversely, rise in concentration of inhibitor resulted to rise in efficiency of inhibitor.Furthermore, it was also observed that at higher temperatures, there were enhanced inhibitor efficiencies for individual concentrations. For example, at 60°C, 0.4g/L inhibitor concentration yielded an inhibitor efficiency of 80.6841%, while at 70°C, 0.4g/L resulted to inhibitor efficiency of 82.7798%. This was observed generally on average. This implies that the inhibitor is more effective at elevated temperatures.From figure 4, the variation of concentration of inhibitor per surface coverage for various inhibitor concentrations was analyzed. It was observed as the concentration of inhibitor increased, the C/ϴ also increased. Hence, at elevated temperatures, Alchornea Cordifolia leaf extract also played optimum role in inhibiting corrosion of mild steel in 0.2M Sulphuric acid. It is crystal clear from the result that as the reaction temperature was increased from 303K to 323K, 333K, and to 343K, the efficiency of inhibition increased. This suggests a physical adsorption mechanism. The plots of C/ϴ against C are linear, showing that Alchornea Cordifolia leaf extract obeys the Langmuir adsorption Isotherm at the concentrations and temperatures studied for mild steel. The various plots support the assertion that the corrosion inhibition mechanism is due to formation and maintenance of a impermeable layer on metal surface and the additives cover anodic and cathodic sites through uniform adsorption following Langmuir Isotherm. The linear regression coefficients between C/ϴ and C show that inhibitor adsorption on mild steel surface agrees with the Langmuir Isotherm [23, 24].The following equations were obeyed:
 | (3) |
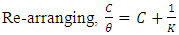 | (4) |
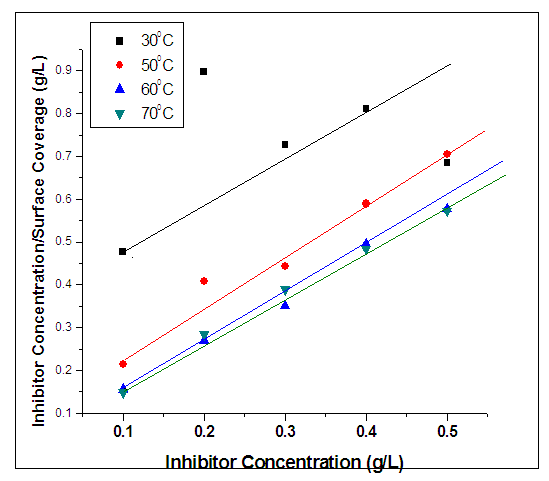 | Figure 4. Langmuir plot for inhibition corrosion of mild steel in 0.2M H2SO4 with Alchornea Cordifolia Leaf Extract at 30°C, 50°C, 60°C, and 70°C for 2Hours |
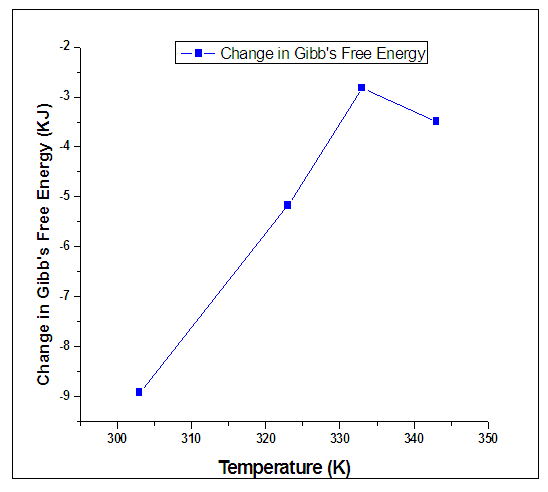 | Figure 5. Variation of Change in Gibb’s Free Energy and Temperature for different Inhibitor Concentrations of Alchornea Cordifolia leaf extract for mild steal in 0.2M Sulphuric acid at 30°C, 50°C, 60°C, and 70°C for 2Hours |
4. Conclusions
- Having analyzed the outcome of the research, we concluded that corrosion rate of mild steel in Sulphuric acid is dependent on Alchornea Cordifolia leaf extract concentration. It means that the level of corrosion inhibition rendered by this extract on the mild steel increases with increase in concentration of the extract and temperature. The inhibitor is more effective at elevated temperatures. Results presented in the table 2 indicate that the values of ∆G are negative in all cases and ranged between -3.49KJ/mol and -8.92KJ/mol. The negative values indicate a spontaneous adsorption of the inhibitor molecules. The values of ∆G are below -40 KJ/mol which indicate that the inhibitor function by physically adsorbing on the surface of the metal. The inhibition is purely by physical adsorption of the active components in the extract on the mild steel surface. Alchornea Cordifolia leaves have tannins as the most active constituent. Tannins can be used for the production of anti-corrosive primer for treatment of rusted steel surfaces prior painting, rust converter to transform oxidized steel into a smooth sealed surface and rust inhibitor. Hence the reason for the inhibition is primarily due to the presence of tannins in the Alchornea Cordifolia leave extract. The data obtained from this study fit well into Langmuir adsorption Isotherm.
References
| [1] | Osarolube E., Owate I. O., Oforka N.C. (2004). “The Influence of Acidic Concentrations on Corrosion of Copper and Zinc” J. Corr. Sci. Tech. 1.1 pp. 66-69. |
| [2] | Farina C.A., Faita G., Olivani F. (2004). “Electrochemical Behaviour of Iron in Methanol and Dimethylformalmide solution”, Corrosion Science (18): 463-479. |
| [3] | Osarolube E., Owate I. O., Oforka N. C. (2004). “The Influence of Acidic Concentrations on Corrosion of Copper and Zinc” J. Corr. Sci. Tech. 1.1 pp. 66-69. |
| [4] | Clark D. S., Varney W. R. (1987). “Physical Metallurgy for Engineers”. Van Nostrand Reinhold Ltd., Canada, pp.300-315. |
| [5] | Osarolube, E., Owate, I. O., and Oforka, N. C. (2008). Corrosion behaviour of mild and high carbon steels in various acidic media. Scientific Research and Essay Vol.3 (6), pp. 224-228. |
| [6] | Callister, W. D., Jr. and Rethwisch, D. G. (2014). Materials Science and Engineering (second edition). Wiley India Pvt Ltd. Pp 580. |
| [7] | Mathiyamsu, J., Nebru, I. C., Subramania, P., Palaniswamy, N., Rengaswamy, N. S. (2001). Anticorros. Methods Mater. 48(5), 342. |
| [8] | Ochao, N., Moran, F., Pebre, N. (2004). Applied Electrochemistry. 34, 487. |
| [9] | Oguzie, E. E., Unaegbu, C., Ogukwe, C. N., Okolue, B. N., & Onuchukwu, A. I. (2004). Inhibition of mild steel corrosion in sulphuric acid using indigo dye and synergistic halide additives. Materials Chemistry and Physics, 84(2-3), 363-368. |
| [10] | Oguzie, E. E. (2004). Influence of halide ions on the inhibitive effect of congo red dye on the corrosion of mild steel in sulphuric acid solution. Materials chemistry and Physics, 87(1), 212-217. |
| [11] | Rajendran, S., Maria Joany, R., Apparao, B. V., Palaniswamy, N. (2000). Trans. SAEST 35(3/4), 113. |
| [12] | Rajendran, S., Apparao, B. V., & Palaniswamy, N. (1998). Synergistic effect of ethyl phosphonate and Zn2+ in low chloride media. Anti-Corrosion Methods and Materials, 45(5), 338-343. |
| [13] | Shalaby, M. N., & Osman, M. M. (2001). Synergistic inhibition of anionic and nonionic surfactants on corrosion of mild steel in acidic solution. Anti-Corrosion Methods and Materials, 48(5), 309-318. |
| [14] | Ebenso, E. E. (2003). Synergistic effect of halide ions on the corrosion inhibition of aluminium in H2SO4 using 2-acetylphenothiazine. Materials Chemistry and Physics, 79(1), 58-70. |
| [15] | Quraishi, M. A., & Sharma, H. K. (2003). 4-Amino-3-butyl-5-mercapto-1, 2, 4-triazole: a new corrosion inhibitor for mild steel in sulphuric acid. Materials Chemistry and Physics, 78(1), 18-21. |
| [16] | Abiola, O. K. and Oforka, N. C. (2002). “Inhibition of the Corrosion of Mild Steel in Hydrochloric Acid by (4- Amino – 2 –Methyl-5-Pyrimidinyl Methylthio) Acetic Acid and its Precursor”, Journal of Corrosion Science and Engineering, vol 3, Pages 1-8. |
| [17] | Abdallah, M., Asghar, B. H., Zaafarany, I., & Fouda, A. S. (2012). The inhibition of carbon steel corrosion in hydrochloric acid solution using some phenolic compounds. Int. J. Electrochem. Sci, 7(1), 282-304. |
| [18] | Abdel-Aal, M. S., & Morad, M. S. (2001). Inhibiting effects of some quinolines and organic phosphonium compounds on corrosion of mild steel in 3M HCl solution and their adsorption characteristics. British Corrosion Journal, 36(4), 253-260. |
| [19] | Abdallah, M., & El-Naggar, M. M. (2001). Cu+ 2 cation+ 3, 5-dimethyl pyrazole mixture as a corrosion inhibitor for carbon steel in sulfuric acid solution. Materials chemistry and physics, 71(3), 291-298. |
| [20] | Bouklah, M., Ouassini, A., Hammouti, B., & El Idrissi, A. (2006). Corrosion inhibition of steel in sulphuric acid by pyrrolidine derivatives. Applied surface science, 252(6), 2178-2185. |
| [21] | Callister, W. D. (1997). ‘‘Materials Science and Engineering’’. 4th ed. John – Wiley, New York, 1997. |
| [22] | www.nace.org retrieved Friday July 6, 2007. |
| [23] | Durowaye, S. I., Durowaye, V. O., Begusa, B. M. (2014). Corrosion of mild steel I acidic medium by methyl Red (2, 4-Dimethylalino-2’-carboxylazobenzene). International journal of engineering and technology. 4:8. 469-470. |
| [24] | Iwu, M.M., (1993). Handbook of African medicinal plants. CRC Press, Boca Raton, Florida, United States. 464 pp. |
| [25] | Kamara, A.Y., Akobundu, I.O., Sanginga, N. & Jutzi, S.C., (2000). Effect of mulch from 14 selected multipurpose trees (MPTs) on growth, nitrogen nutrition and yield of maize (Zea mays L.). Journal of Agronomy and Crop Science 184: 73–80. |
| [26] | Kang, B.T., Caveness, F.E., Tian, G. & Kolawole, G.O., (1999). Longterm alley cropping with four hedgerow species on an Alfisol in southwestern Nigeria - effect on crop performance, soil chemical properties and nematode population. Nutrient Cycling in Agroecosystems 54: 145– 155. |
| [27] | Kanmegne, J., Duguma, B., Henrot, J. & Isirimah, N.O., (1999). Soil fertility enhancement by planted tree-fallow species in the humid lowlands of Cameroon. Agroforestry Systems 46: 239–249. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML