-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2018; 8(3): 45-50
doi:10.5923/j.materials.20180803.01

Acidic Corrosion Inhibition Mechanism of Aluminum Alloy Using Green Inhibitors
Francis O. Nwosu1, 2, Israel O. Owate1, E. Osarolube1
1Physics Department, University of Port Harcourt, Nigeria
2Physics Electronics Department, Abia State Polytechnic, Aba, Nigeria
Correspondence to: Francis O. Nwosu, Physics Department, University of Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

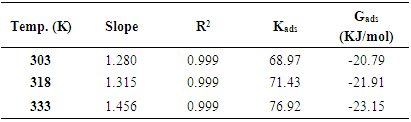
The corrosion inhibition mechanism of Al alloy AA5052 was studied using gravimetric technique and potentiodynamic polarization in 0.5 M HCl. Reflux extraction technique was used on Newbouldia laevis leaf extract. The weight loss and the inhibition efficiency were calculated. The density current was of the inhibition system were reduced. An optimal inhibition efficiency of 87% was obtained. The green leaf extract was observed adsorbed on the surface according with Langmuir and Temkin adsorption isotherm models. The effects of temperature variations which led to thermodynamic parameters were studies. The negative adsorption energy, ∆Gads obtained suggested a spontaneous molecules adsorption on the metal surface. Light Micrograph was employed to study the surface morphology. The mechanical hardness strength of the metal was improved due to the protection of the leaf extract.
Keywords: Corrosion studies, Gravimetric, Potentiodynamic Polarization, Thermodynamic parameters and Green Corrosion Inhibition
Cite this paper: Francis O. Nwosu, Israel O. Owate, E. Osarolube, Acidic Corrosion Inhibition Mechanism of Aluminum Alloy Using Green Inhibitors, American Journal of Materials Science, Vol. 8 No. 3, 2018, pp. 45-50. doi: 10.5923/j.materials.20180803.01.
Article Outline
1. Introduction
- There seemed to be ongoing fight between man and nature seen in corrosion and corrosion prevention. The need to preserve materials has been a great concern to scientists and engineers knowing the harmful effects of corrosion are an undesirable phenomenon. Thus the need for green corrosion inhibition since the use of chemical/synthesized inhibitors failed due to its hazardous effects. The use of natural inhibitors has been one of the best supported approaches to corrosion inhibition in recent times [1-3]. Green inhibitors (such as the roots, seeds, leafs, etc) are preferred due to their comparative advantage. They are cheap, eco-friendly, non-toxic, [4] and they contain compounds like nitrogen, sulfur and oxygen which are organic heterocyclic compounds and contain pi (π) electrons polar groups.Acids are widely used in various industries for pickling metals. They are also used in oil and gas flow to disqualify encrustations in production wells [4]. We also surfer from acidic rain fall and metals exposed to work suits enabling environment for corrosion attacks. Among various acids, hydrochloric acids are mostly used for this purpose [5, 6].Nwebouldia laevis is a shrub belonging to the family nignoniaceae. It is used healing pain, arthritis, rheumatism, diarrhoea, oedema, spasm bark, etc [7] they contains the following phytochemicals: tannins, alkaloids, saponnins and flavoniods. The presence of these constituents makes it suitable for corrosion inhibition considerations.
2. Materials and Methods
2.1. Newbouldia laevis Leaf Preparations
- The leaf of the newbuldia laevis (NB) were collected from Niger Delta region of Nigeria. Air dried away from direct sunlight and prepared according to [8-11] under reflux for 3 hours at constant heat supply (70 °C). The extract is then further diluted into the various concentrations: 0.0, 0.1, 0.2, 0.3, 0.4, and 0.5 g/L using 0.5 M HCl solution.
2.2. Metal Preparations
- The aluminium alloy AA5052 used in this study was analysed with optical emission spectrometry (OES) and contains the following (in %weight.): Al(98.990), Zn(0.08), Ti(0.583), Cr(0.760), Fe(0.221) and other trace elements. The metal sheets were cut into coupons of dimension (20 x 20 x 1.5 mm), washed with detergent, degreased in ethanol and air dried before weighing.
2.3. Gravimetric Technique and Electrochemical Measurements
- The pre-weighed coupons were exposed to different test solutions. The study was conducted with the different concentrations of the inhibitor at varied temperatures. After the exposure period, the coupons were retrieved, rinsed with plenty water dried thereafter weighed. The weight loss determined. The procedure was done in duplicate and the average result taken for reproducibility.The electrochemical measurements have been described in details [11-14]. Electrochemical tests were conducted using a PAR-2273 Advanced Electrochemical System workstation using standard three electrode electrochemical cell. The working electrode is aluminum AA5052. A saturated calomel electrode (SCE) was used as the reference electrode, while a graphite rod was used as the counter electrode. A metal specimen fixed in epoxy resin with a surface area of 1.0 cm2 exposed to the test solution, served as the working electrode. Electrochemical measurements were carried out in aerated and unstirred solutions at the end of 3600 s of immersion, which allowed the Open Circuit Potential (OCP) values to attain steady state. Temperature was fixed at 30±1°C. Potentiodynamic polarization studies were conducted in the potential range +250 to – 800 mV versus corrosion potential at a scan rate of 0.333 mV/s. Each test was run in triplicate to verify the reproducibility of the systems.
2.4. Mechanical Properties
- The Hardness mechanical tests were performed on the without surface preparation just as retrieved from the test solutions. A load of 10 N was used. The Vickers microindentation tests were performed on the raw coupons. Five different indentations were made on the coupons and the average value reported.
3. Results and Discussions
3.1. Influence of Inhibitor Concentration on the Weight Loss, Corrosion Rate and Inhibition Efficiency
- In the absence and presence of newbuldia laevis (NB), the weight loss (figure 1) showed that the presence of the leave extract significantly decreased the mass degraded from the metal. The weight loss is found to slightly increase for the cases of the inhibited systems. Figure 2 interestingly showed the rate of corrosion attack on the Al alloy. The corrosion rate was calculated using eq. 1. [15].
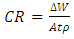 | (1) |
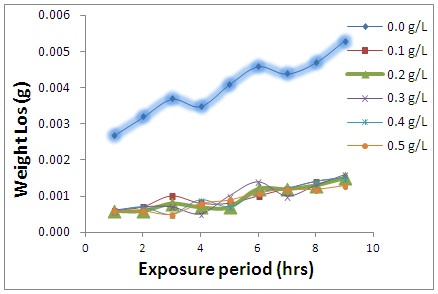 | Figure 1. Weight loss variations of AA5052 in 0.5 M HCl in the presence of Newbouldia leavis extract |
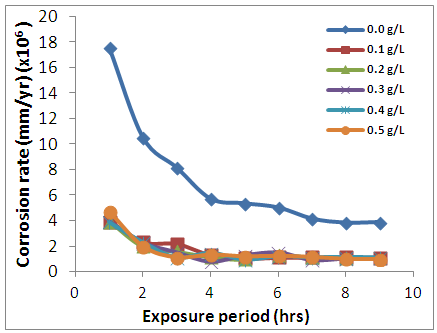 | Figure 2. Corrosion rate variations of AA5052 in 0.5 M HCl in the presence of Newbouldia leavis extract |
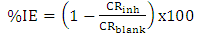 | (2) |
 | Figure 3. Inhibition efficiency variation of Newbouldia leavis extraction AA5052 in 0.5 M HCl solution |
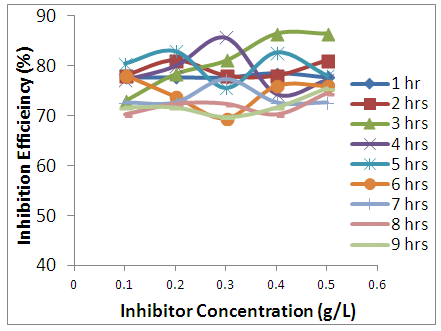
 | Figure 4. Inhibition efficiency variation against inhibitor concentration at varies temperatures |
3.2. Electrochemical Impedance Spectroscopy (EIS) Measurement
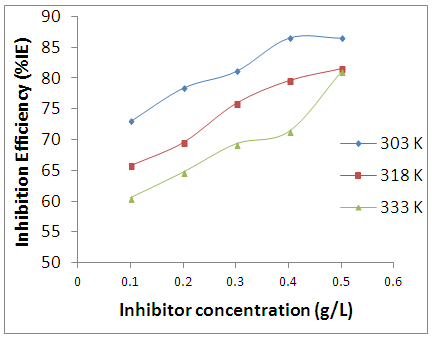
- The impedance method provides information about the kinetics of the electrode processes as we all the surface properties of the investigated system [16]. The shape of the impedance gives information about the mechanism of corrosion inhibition [17]. The EIS measurements were carried out to study the corrosion of AA5052 alloy in 0.5 M HCl in the presence and absence of different concentrations of Newbouldia laevis extract. The Nyquist plot (figure 5) showed spectrums which is characterised by semi-circle. The diameter of the semicircle for the inhibitive system is larger thus it has more capacitive loop with low frequency dispersion (inductive arc). A capacitive loop arises from the time constant of the electric double layer and charge-transfer resistance, and an inductive loop appeared at Low-frequency (LF) values in the presence of Newbouldia laevis extract. The presence of the LF inductive loop may be attributed to the relaxation process obtained by adsorption the π-electrons of the conjugated systems O- on the electrode surface [17-20]. It may also be attributed to adsorption of inhibitor on the electrode surface [21] which creates barrier to current flow or to charge-transfer. Rs represent the charge transfer resistance. It measures the electron transfer across the surface, which is inversely proportional to the corrosion rate [22].
 | Figure 5. Nyquist plots for AA5052 in 0.5 M HCl at different inhibitor concentrations of the Newbouldia laeis at room temperature |
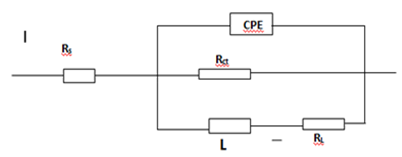 | Figure 6. The equivalent model circuit of the impedance spectra |
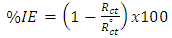 | (3) |
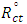 are the charge-transfer resistance for the inhibited system and the inhibitor-free system.
are the charge-transfer resistance for the inhibited system and the inhibitor-free system.3.3. Adsorption Models and Thermodynamic Considerations
- Adsorption at the metal-solution interface has been considered as the primary step in the action of most organic corrosion inhibitors in acidic environment. Basic information on the adsorption of inhibitors on the metal surface can be provided by adsorption isotherm. In order to obtain the isotherm, the fractional surface coverage values θ as a function of inhibitor concentration must be obtained. The values of θ can be easily determined from the weight loss and electrochemical measurements by the ratio %IE/100, where %IE is inhibition efficiency obtained by weight loss and thermometric measurement [25]. Langmuir adsorption isotherm (equation 4) was used to deduce whether there is formation of layer of insoluble complex of the metal on the surface which acts as a barrier.
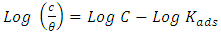 | (6) |
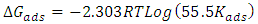 | (7) |
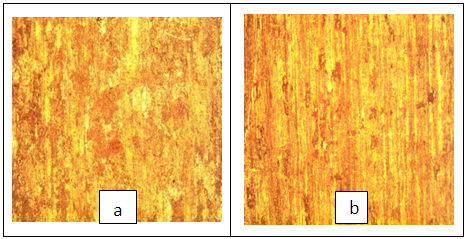 | Figure 7. Micrograph of AA5052 alloy immersed in 0.5 M HCl without inhibitor (a) and with Newbouldia laevis extracts (b) |
|
3.4. Surface Morphology
- The light micrographs of the AA5052 immersed in 0.5 M HCl solution with and without inhibitor are presented in figure 8. The micrographs reviewed the micrograph without inhibitor showed high density of porosity. The yellowish nature of the micrograph shows that the parent element Aluminum was attached greatly. This is recognized because of the effect of the corrosion attack. However, figure 7b showed that there is reduction of the yellowish patches. This showed certain level of barrier creation which seen as patches of films. This spots that the nature of adsorption of the inhibitor is physical. It is possible that the parent metal must have formed thin film oxide (aluminum oxide) the process called passivation. The thin films could as well enhance the level of barrier creation.
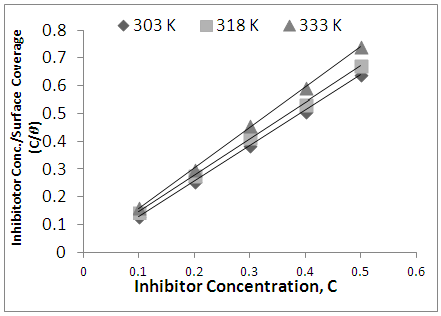 | Figure 8. Langmuir Adsorption Isotherm model of Newouldia Laevis on AA5050 Surface in 1.0 M HCl |
3.5. Mechanical Hardness Test
- The Vickers hardness test conducted on the Alloy exposed to the various corrosion test solutions were reported in figure 9. The Raw coupon showed highest hardness strength. The corrosion attack greatly reduced the hardness of the AA5050 alloy when exposed to inhibitor-free environment. But the presence of the newbouldia laevis extract improved the hardness ability of the corroded AA5050 alloy.
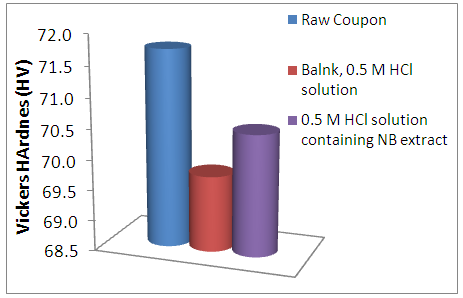 | Figure 9. Mechanical Vickers microindentation value of AA5052 exposed to acidic environment |
4. Conclusions
- The presence of Newbouldia laevis extract in 0.5 M HCl solution reduced the corrosion rate of AA5052. The inhibition efficiency increases with increase in the concentration, but decreases with rise in temperature. The inhibition process obeys Langmuir adsorption model and it was found to be through physical adsorption. The Gibb’s free energy of adsorption ΔG at all temperature exposure indicates that the adsorption process was spontaneous. The electrochemical impedance spectroscopy (EIS) result revealed that the presence of the inhibitor developed an increase in double layer on the metal surface. This limits the interactions between the surface of the metal to and the corrosive environment. Also the mechanical hardness properties of AA5052 were improved after corrosion attack due to the presence of the green inhibitor.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML