-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2018; 8(2): 39-44
doi:10.5923/j.materials.20180802.03

Adsorption and Anticorrosion Performance of Ocimum Canum Extract on Mild Steel in Sulphuric Acid Pickling Environment
N. B. Iroha, A. Hamilton-Amachree
Department of Chemistry, Federal University, Otuoke, Nigeria
Correspondence to: N. B. Iroha, Department of Chemistry, Federal University, Otuoke, Nigeria.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The corrosion inhibitory effect of extracts from Ocimum Canum (OC) on mild steel was studied in 0.5 M H2SO4 using gasometric and weight loss methods of monitoring corrosion. The result shows that the OC extract functioned as an effective inhibitor for mild steel corrosion in H2SO4 solutions. The inhibition efficiency of OC on mild steel corrosion in 0.5 M H2SO4 solution increases on increasing its concentration but decreases when temperature is increased. The inhibition is attributed to the adsorption on the mild steel surface by constituents of OC extract and conforms to Langmuir adsorption isotherm. Mechanisms of inhibition were deduced using the dependence of the inhibition efficiency on temperature and also from assessment of kinetic and activation parameters which govern the processes and it was discovered that the adsorption of the extract’s organic constituents on the corroding mild steel were by physical adsorption.
Keywords: Ocimum Canum, Corrosion Inhibitor, Mild Steel, Gravimetric, Gasometric, Physical adsorption
Cite this paper: N. B. Iroha, A. Hamilton-Amachree, Adsorption and Anticorrosion Performance of Ocimum Canum Extract on Mild Steel in Sulphuric Acid Pickling Environment, American Journal of Materials Science, Vol. 8 No. 2, 2018, pp. 39-44. doi: 10.5923/j.materials.20180802.03.
Article Outline
1. Introduction
- In chemical industries, acidic solutions are usually used for industrial cleaning and to remove scales from metal surfaces, these processes lead to significant metal dissolution. In order to prevent and minimize the dissolution of metals in acidic environments, different corrosion inhibitors are used, especially organic compounds, which in their structure contain some of the following atoms; nitrogen, sulphur, oxygen, phosphor etc. [1-4]. Most corrosion inhibitors that are synthesized are usually hazardous and this has favoured using environmentally acceptable inhibitors. Natural products are renowned corrosion inhibitors, being environmentally friendly. The extracts from various parts of some plants have been revealed to inhibit corrosion of mild steel in acidic solutions [5-8]. These natural inhibitors studied have been discovered to be highly eco-friendly and possess no threat to the environment.Ocimum Canum (OC) belongs to the Lamiaceae family. It is also called the African basil with a distinct mint flavor, with hairy leaves and scented flowers. OC was used specially for treating diabetes. OC is common in Africa, usually in the tropics and other areas of the equatorial region and was after some time introduced to the Americas. The stems of the plant are angled and the branches are from its base and the leaves are oval. The leaves are fuzzy and tiny and have flowers which are beautiful violet or white, with a pleasant scent like that of a clove. The leaves are opposite and toothed [9].The present study is directed at the evaluation of extracts from OC as an inhibitor for the corrosion of mild steel in sulphuric acid pickling environment employing gasometric and weight loss techniques. Phytochemical screening of the extract of the inhibitor reveals that the OC extract contains alkaloid, saponin, anthraquinone, tannin, phlobatanins, steroids, flavanoids, among others [10].
2. Materials and Methods
2.1. Material Preparation
- Mild steel sheets with weight percentage composition as follows: C, 0.05; Mn, 0.6; P, 0.36; Si, 0.03 were used. The sheets with thickness 0.14 cm, was pressed-cut mechanically into coupons 2cm × 4cm dimension. These coupons were utilized as procured without polishing, but were however degreased in ethanol, dried with acetone, weighed and kept in a desiccator before use [11].
2.2. Inhibitor Preparation
- Weighed quantity of the dried and ground OC were refluxed with ethanol for about 4 hours and was kept overnight to completely extract the basic components. The resulting solutions were filtered and the ethanol evaporated followed by drying in an oven for one night. The solid extract was stored after weighing and used as inhibitor for the corrosion study. From the weighted solid extract, test solutions of the inhibitor were prepared in the concentration range 0.1 – 2.0 g/L.
2.3. Weight Loss Measurement
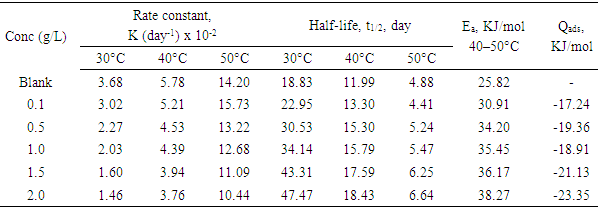
- Tests were carried out by totally immersing the coupons into the test solutions and maintained at 30, 40 and 50°C. The previously cleaned and weighed mild steel coupons were hung with glass hook and rod inside beakers containing inhibitor test solutions. The entire test was conducted in solutions that are aerated. To determine weight loss against time, the mild steel coupons were removed from the test solution after every 24 hours progressively for a period of 120 hours, scrubbed with brush and rinsed with water, dried with acetone and re-weighed. The difference in weight between the coupons at a particular time and the initial weight of the coupons were taken to be the weight loss. From the data gotten from weight loss, the corrosion rates (CR) were calculated from equation (1):
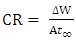 | (1) |
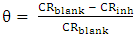 | (2) |
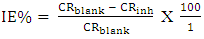 | (3) |
2.4. Gasometric Measurement
- Gasometric measurement was carried out by using the gasometric assembly which is similar to the set-up described by Oguzie [12]. It consist basically two necked round-bottom flask which serves as the medium for the reaction containing the metal coupons and the corrodent. Other parts are a separating funnel, a burrete fitted with taps, and an outer glass jacket which serves as a water condenser. A two-necked round-bottom flask was connected to a burette through a delivery tube which was also connected to a reservoir containing paraffin oil. About 200ml of the test solution was then introduced inside the flask and the initial volume of air in the burette recorded. Thereafter, the reaction vessel was immediately closed after two coupons were dropped in the solution. The change in volume of the paraffin oil level was used to estimate the volume of hydrogen gas evolved by the corrosion reaction. The evolved gas was carefully measured at fixed time interval to monitor the progress of the corrosion reaction. Experiments were carried out at 30°C, 40°C and 50°C. The hydrogen evolution rate (RVH) is determined from the ratio of the volume of evolved gas (V) to time (t), according to equation (4)
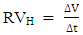 | (4) |
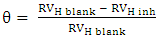 | (5) |
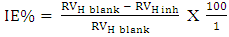 | (6) |
3. Results and Discussion
3.1. Weight Loss Experiment
- The plot of the rate of corrosion and efficiency of the inhibitor with inhibitor concentration for mild steel corrosion in 0.5 M H2SO4 at 30°C, 40°C and 50°C is shown in Figure 1 and 2. It is evident from Fig. 1 that the rate of corrosion decreased in the presence of the inhibitors compared to blank solution. This clearly indicates that OC extract retarded mild steel corrosion in H2SO4 environment. It is also noticed that corrosion rate is increased as the temperature is elevated from 30 to 50°C both in the presence and absence of the inhibitors. This shows that the metal is susceptible to faster dissolution with increasing thermal agitation of the corrosive environment [13]. From Figure 2, it is observed that inhibition efficiency increased with an increase in OC extract concentration. The increase in inhibition efficiency and the decrease in corrosion rates with the increase in inhibitor concentration could be ascribed to the adsorption of the molecules of the inhibitor on the mild steel surface. The surface coverage occupied by the adsorbed molecules increases by increasing the concentration of the inhibitor which leads to better protection.
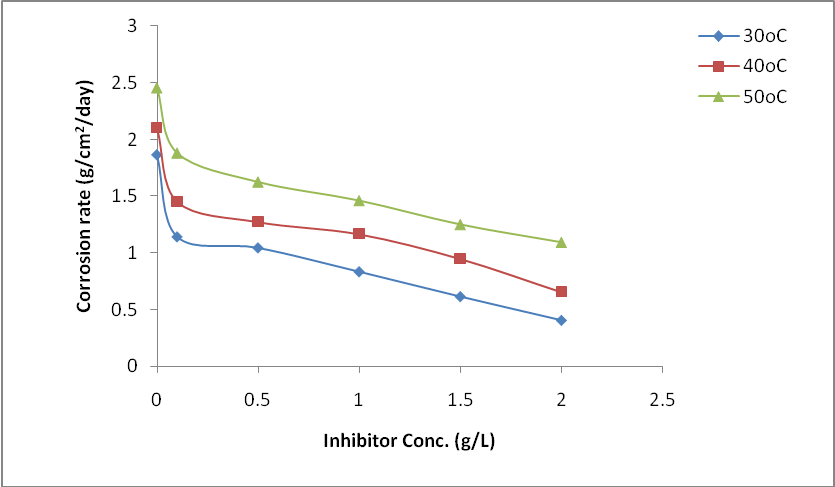 | Figure 1. Variation of corrosion rate with concentration for mild steel with and without different concentrations of OC in 0.5 M H2SO4 solution at different temperatures |
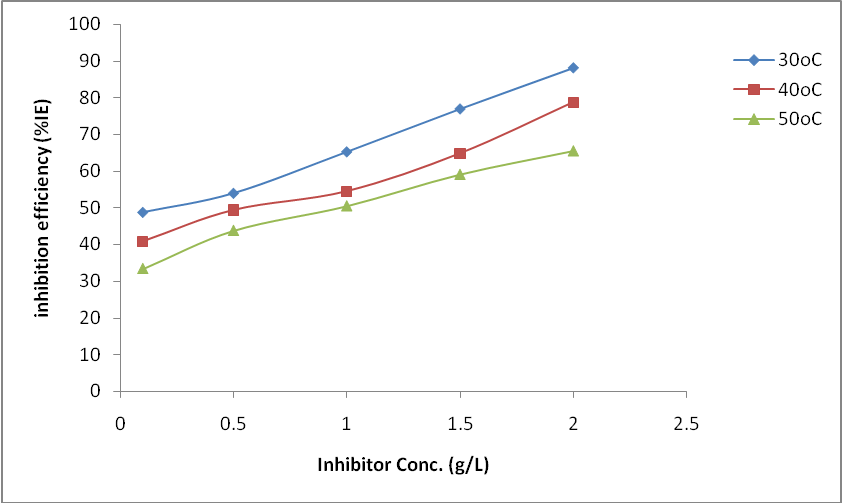 | Figure 2. Variation of inhibition efficiency (%IE) with OC concentration for mild steel in 0.5 M H2SO4 solution at different temperatures from gravimetric measurements |
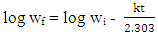 | (7) |
|
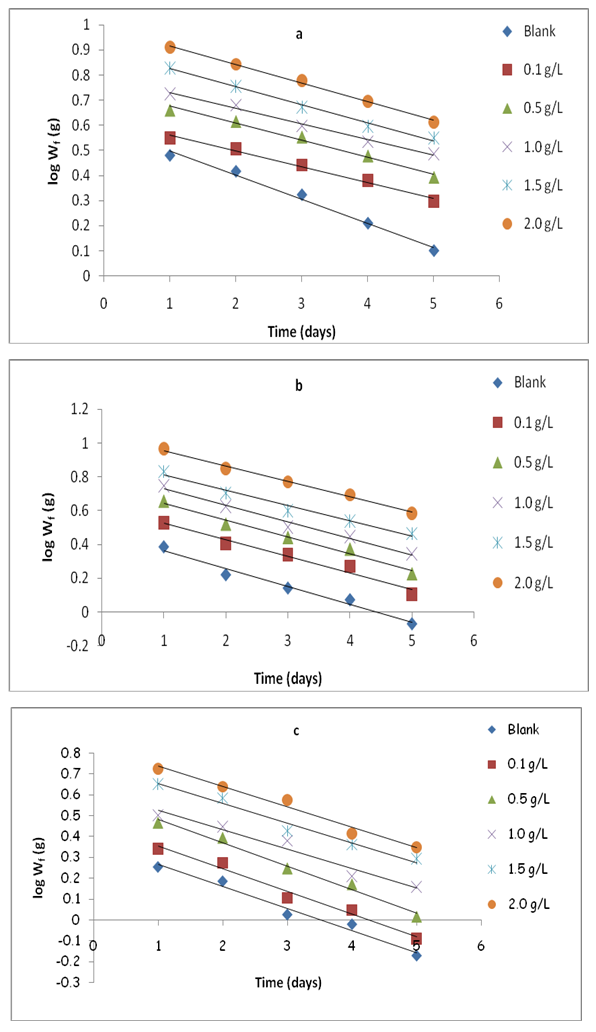 | Figure 3. Variation of log Wf with time for mild steel in 0.5 M H2SO4 solution containing various concentrations of ethanol OC extract at (a) 30°C (b) 40°C and (c) 50°C |
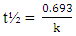 | (8) |
3.2. Gasometric Measurement
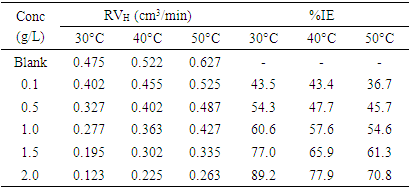
- Fig. 4 shows the variation of hydrogen gas evolved rate with inhibitor concentration for mild steel corrosion in 3 M H2SO4 containing different concentrations of OC extract at different temperatures. The figure revealed higher rate of evolution of hydrogen gas by the blank solutions as compared to the evolution by solutions containing different concentrations of OC extract indicating that OC extract functioned as an inhibitor for mild steel corrosion in H2SO4 at the studied temperatures. It is equally observed that the hydrogen gas evolution rate decreases with an increase in the concentration of OC extract.
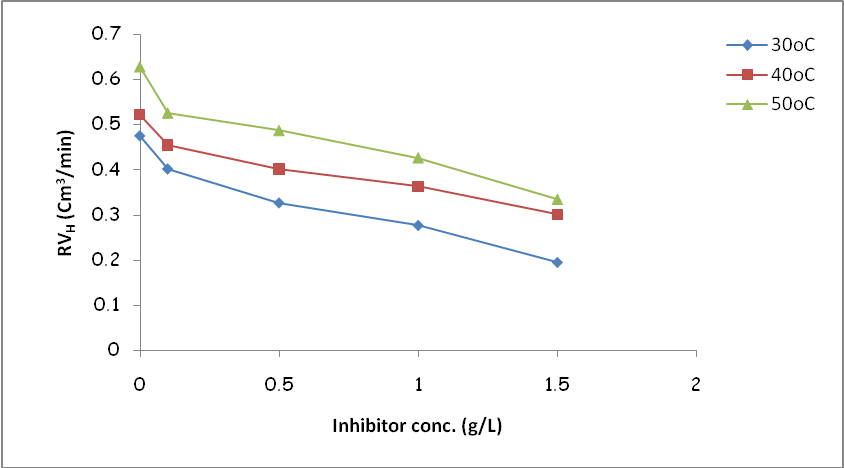 | Figure 4. Variation of rate of hydrogen gas evolved with inhibitor concentration for mild steel corrosion in 3 M H2SO4 containing different concentrations of OC extract |
3.3. Effect of Temperature
- Evaluating the effect of temperature on the rate of corrosion and inhibition efficiency of OC extract in H2SO4 has given insight into the stability and adsorption mechanism of the inhibitor on the surface of the mild steel. From weight loss measurement, it is observed from Fig. 1 that at all of the temperatures studied the rate of corrosion increases with temperature rise. However, inhibition efficiency of the OC extract decreases as the temperature increases. This trend is consistent with both gasometric and weight loss methods employed in this study as presented in Fig. 1 and Table 2 respectively. Decrease in inhibition efficiency with temperature rise is suggestive of physical adsorption of OC extract onto the surface of the mild steel [15].
|
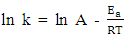 | (8) |
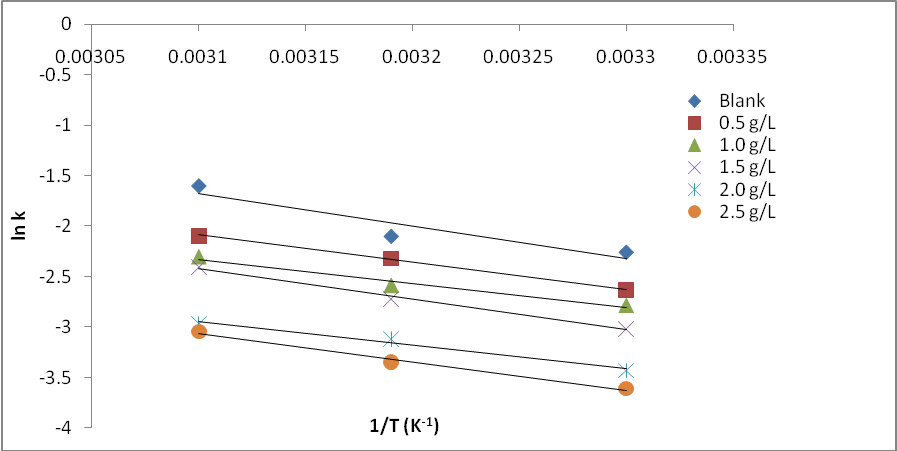 | Figure 5. Arrhenius plots for the corrosion of mild steel in 0.5 M H2SO4 with and without different concentrations of OC |
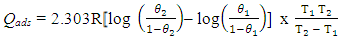 | (9) |
3.4. Adsorption Behaviour
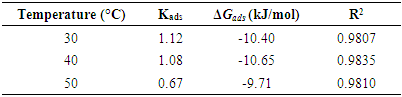
- To further elucidate the adsorption behaviour of OC extract in 0.5 M H2SO4 solution, different isotherm models were employed, such as Freundlich, Langmuir, Flory-Huggins, Frumkin, and Temkin, but the best fit was the Langmuir isotherm model as presented in Figure 6, obtained by using the weight loss data. The Langmuir adsorption isotherm is represented by equation 10:
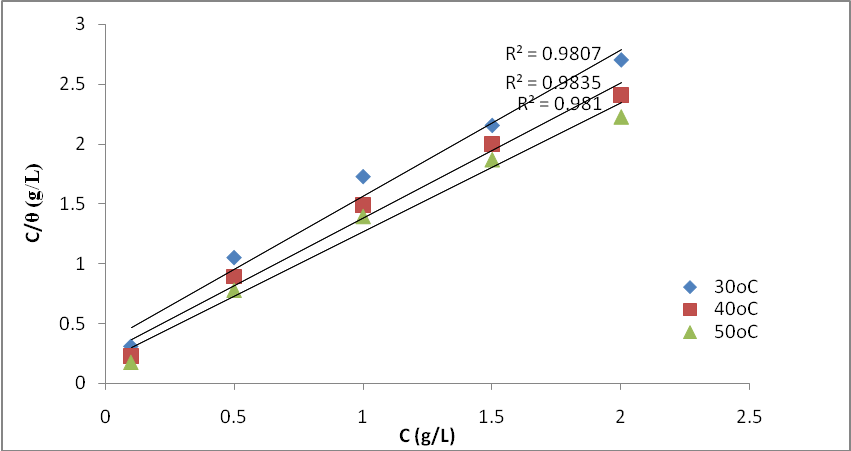 | Figure 6. Langmuir Isotherm for the adsorption of OC extracts on mild in 0.5 M H2SO4 at different temperatures |
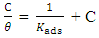 | (10) |
|
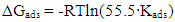 | (11) |
4. Conclusions
- The results presented in this paper show that Ocimum Canum (OC) inhibits mild steel corrosion in H2SO4 solutions to reasonable extent. The corrosion rate of the mild steel in 0.5 M H2SO4 is dependent on the concentration of the inhibitor. This rate decreased as the concentration of the inhibitor is increased. The percentage inhibition efficiency of this inhibitor decreased with temperature rise which indicate that physical adsorption was the predominant inhibition mechanism. OC extract can be considered as an eco-friendly corrosion inhibitor for mild steel in 0.5M H2SO4 solution.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML