-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2018; 8(2): 32-38
doi:10.5923/j.materials.20180802.02

Cationic Dyes Removal Using Low-Cost Banana Peel Biosorbent
Muntaka Dahiru1, Zakariyya Uba Zango2, Maje Alhaji Haruna3
1Department of Science Laboratory Technology, School of Technology, Kano State Polytechnic, Kano, Nigeria
2Department of Chemistry, Al-Qalam University, Katsina, Nigeria
3Department of Chemistry, Federal University, Dutse, Jigawa, Nigeria
Correspondence to: Muntaka Dahiru, Department of Science Laboratory Technology, School of Technology, Kano State Polytechnic, Kano, Nigeria.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The applicability of banana peel as low cost adsorbent for the removal of cationic Malachite green (MG) and Methylene from aqueous solution has been studied. Banana peel, like other biomass has high content of carbohydrate and fiber with different functional groups such as hydroxyl (OH), carboxylic (COOH) and amines (NH2). This has been confirmed from the spectrum obtained from FTIR analysis. Batch adsorption studies were carried out to determine the effect of contact time and adsorption capacity of the biosorbent. Equilibrium was attained within 120 mins with equilibrium adsorption capacity (qe) of 107±1.3 mg/g and 120±2.7 mg/g for MG and MB cationic dyes respectively with a stirring rate of 200 rpm. Results from the effects of adsorbent dosage and initial dye concentrations have shown that 0.2 g of the adsorbent and 50 mg/g of the dye solutions were the optimum amounts for efficient removal of the dyes. The adsorption capacities were shown to be favored at high pH of the medium due to the high interactions between the surface of the adsorbent and the cationic dyes. From the economic point of view, regeneration and reusability of the adsorbent is a good indicator of its applicability as low-cost biosorbent for cationic dyes remediation in industrial wastewater.
Keywords: Adsorptions, Banana peel, Malachite, Methylene blue, Removal
Cite this paper: Muntaka Dahiru, Zakariyya Uba Zango, Maje Alhaji Haruna, Cationic Dyes Removal Using Low-Cost Banana Peel Biosorbent, American Journal of Materials Science, Vol. 8 No. 2, 2018, pp. 32-38. doi: 10.5923/j.materials.20180802.02.
Article Outline
1. Introduction
- Environmental contamination of various water compartments with toxic organic compounds such as synthetic and natural dyes, pigments, pesticides, etc. has been a major challenge to the living organisms including human being, aquatic organisms and plants. The major applications of dyes are modifications of color and attribution of unique characteristic to different substrates as paper, fabric, leather, etc [1, 2]. A wide variety of dye molecules are released from textile manufacturing, paper and cosmetic industries, which enter the aquatic compartments through the wastewater effluents. Among the major threats of the dyes molecules is their effect on photosynthesis [3] and are known to cause various health effects to human beings such as skin diseases, breathing difficulties, eyes burn, vomiting, nausea etc. [4, 5]. Moreover, many dyes are toxic and even carcinogenic, thus, affecting the aquatic biota and human health [6, 7]. As such it is paramount to remove the dyes before discharging the water to the environment. The use of agricultural waste materials as adsorbents for dyes removal has gain profound applications. Researchers have focused on those materials because they offer the advantages of efficiency, low-cost and environmental non-toxicity. Some of the biomass that have been employed for dyes treatments include; wheat straw [8] wheat shells [9], rice hulls [10], cashew nut shell [11], banana stalk [12] and groundnut shell [13]. Others include wood apple shell [14], oil palm wood [15], oil palm fiber [16-18] cocoa pod husks [19], orange peels [20, 21] rice husks [22-24], fly ash [25], saw dust [26] activated carbon [27, 28], coconut shell [23] and maize stem tissue [29]. This work is aimed at determining the potentiality of banana peel, (Banana being a native and abundant fruit in Nigeria) as biosorbent for the removal of cationic Malachite green (MG) and Methylene blue (MB) dyes from a solution. Banana peel could serve as adsorbent for dye removal due to its chemical compositions which contained high amount of cellulose, pectin, hemicellulose and lignin and contained various polar functional groups, including hydroxyl, carboxylic and phenolic acid groups [30-32].
2. Materials and Methods
- Banana fruits (Musa sapientum) were purchased at Na’ibawa Market in Kano state Nigeria in May 2017. Malachite green and Methylene blue dyes were purchased from Sigma Aldrich (USA). The sulfuric acid, hydrochloric acid and sodium hydroxide were obtained from Sinopharm chemical Reagent Co., China. All reagents used in this work are of Analytical grade and high purity. Distilled water was used throughout the work.
2.1. Preparation of the Adsorbent
- The peels from the Banana fruits were removed and subjected to air drying for a period of 5 days. They were oven dried for a 24 hours at 50°C. The sample was then grinded into powdered and sieved using 75 μm sieve. The sample was then stored in an airtight polythene container for analysis.
2.2. Determination of Point of Zero Charge of the Adsorbent
- Point of zero charge (pHzpc) for the banana peel was determined for the dried grounded sample. To a series of 100 mL Erlenmeyer flask, 50 mL of distilled water was transferred. Either 0.1 M NaOH or 0.1 M HCl was used to adjust the initial pH values from 2-10. Then 0.5 g of the dried sample was added to each solution. The suspensions were subjected to water bath shaken for a period of 24 hours. The new pH values of the supernatant liquids were then recorded.
2.3. Characterizations
2.3.1. FTIR Analysis
- The Infrared spectra of the banana peel sample for the determinations of functional groups presents were recorded with Perkin–Elmer FT-IR spectrometer (KBr pellets) Model spectrum GX in the region of 4000 cm-1 - 600 cm-1.
2.3.2. SEM Analysis
- Scanning electron microscopy (SEM) was achieved using JEOL JSM-6380LA, Japan, operated at 20 kV to determine the surface morphology of the banana peel. Prior to the analysis the sample was degassed under vacuum and then mounted on adhesive carbon tape attached to an aluminum stub. Coating of the sample was performed with platinum metal using sputter coater.
2.4. Mineral Content Determination
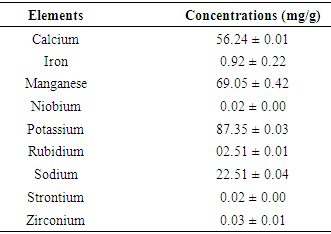
- For the determination of minerals content in the sample, an inductively coupled plasma optical emission spectrometer (ICP OES) with an axially viewed configuration (VISTA PRO, Varian, Mulgrave, Australia) that was equipped with a solid-state detector, Stumar-master mist chamber, and V-groove nebulizer was employed. The sample was digested with Nitric acid and droplets of hydrogen peroxide with a microwave drying.
2.5. Batch Adsorption Experiments
- Batch adsorption experiments were conducted by agitating 0.2 g of the adsorbent with 50 ml of 100 mg/L dye solution at stirring speed of 200 rpm on an thermostat shaker water bath. The aliquots of the dyes were collected at 20 minutes interval, centrifuged at 2000 rpm for 5 min, filtered and unabsorbed supernatant liquid was analyzed for the residual dye concentration using (Varian CARY 50 probe) fitted with a quartz cell with a path length of 1.0 cm. The absorbance measurement of the MG and MB were conducted at 620 and 670 nm respectively. Various concentrations of each dye from 10-50 mg/L were prepared and the calibration curves were plotted to determine the concentrations. The Percentage Removal (%R) is calculated from the formula;
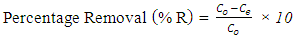 | (1) |
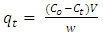 | (2) |
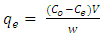 | (3) |
2.6. Effect of Stirring Rate
- The effect of stirring speed was investigated by carrying out adsorption experiment at different stirring rate between 100 - 300 rpm with an initial dye concentration 50 mg/L and the adsorbent dosage of 0.2 g.
2.7. Effect of pH on Dyes Adsorption
- The effect of pH on the uptake of dyes was investigated with the initial dyes concentration of 50 mg/L and the adsorbent dosage of 0.2 g. 0.1 M HCl and 0.1 M NaOH were used to adjust the pH values to form a series of pH from 2 to 12. The suspensions were shaken at room temperature at a speed of 200 rpm.
2.8. Effects of Adsorbent Dosages
- Effects of the adsorbent dosage for the removals of MG and MB were investigated by using various weight ranging from 0.1 – 0.5 g. Initial dye concentrations of 50 mg/L were used at constant stirring speed of 200 rpm.
2.9. Effects of Adsorbates Concentrations
- Effect of changing the concentration of the dyes was investigated because it is essential to determine the threshold limit values of adsorption for possible application. In relation to this, adsorption experiment was carried out to get the equilibrium adsorption capacity and the removal efficiency of the dyes. The concentrations was varied from 50, 100, 150 and 200 mg/L. Experiments were conducted in conical flasks (250 mL) filled with various dyes concentrations (50 mL) and placed on an shaker (200 rpm) at room temperature. At certain intervals of time, aliquots of the sample were taken and their absorbencies were measured at 620 nm and 670 nm for MG and MB respectively to determine the residual dye concentrations.
2.10. Reusability Experiment
- To study the regeneration and reusability of the adsorbent, batch adsorption experiments were conducted with adsorbent dosage of 0.2 g and initial dye concentration of 50 mg/L at room temperature with a stirring rate of 200 rpm. The adsorbent was recovered after the experiment through centrifugation and filtration, followed by washing with 0.1 NaOH and distilled water and dried. Same procedure was repeated 5 times.
3. Results and Discussion
3.1. Characterizations
- FTIR spectrum is used to qualitatively examine the nature of surface and the functional groups which are present in a given materials. Figure 1 represents the typical FTIR of banana peel. Various peaks were observed which indicates the presence of multiple functional groups in the biosorbent. A broad peak at 3640 cm-1 is due to the free hydroxyl group of polymeric compounds [33]. The bond around 2960 cm-1 denotes OH stretching of amino acid groups. The bond between 3000 cm-1 – 2800 cm-1 is due to C-H stretching in alkanes. The observed peak at around 1700 cm-1 is attributed to C=O stretching vibrations of carboxylic groups (-COOH, -COOCH3) which can be due to the presence of carboxylic acids or their esters [34]. The peak at around 1580 cm-1 is assigned to N-H bending vibrations. The peak at 1460 cm-1 is as a result aromatic ring vibration of lignin. Peaks observed at 1364 cm-1 – 11248 cm-1 can be attributed to C-H bending of crystalline cellulose and C-H bending of cellulose, hemicelluloses or lignin polymer. Weak bands observed at around 800 cm-1 – 600 cm-1 were attributed to amines groups. Running FTIR is essential in that it exposes the functional groups that could be responsible for the excellent sorption properties of the material and the spectrum (FTIR) of the biomass has shown that the biosorbent is complex in nature. The presence of various peaks due to different functional groups indicates the complex nature of the biomass and the tendency to acts as a good biosorbent.
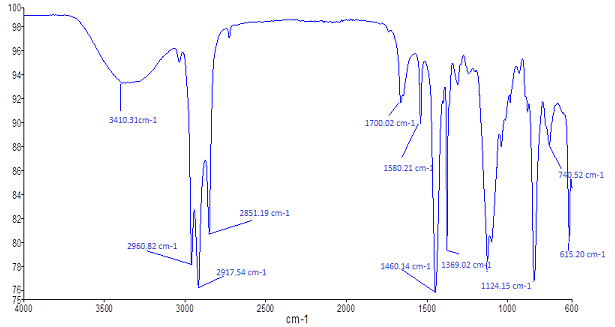 | Figure 1. FTIR spectrum of banana peels |
|
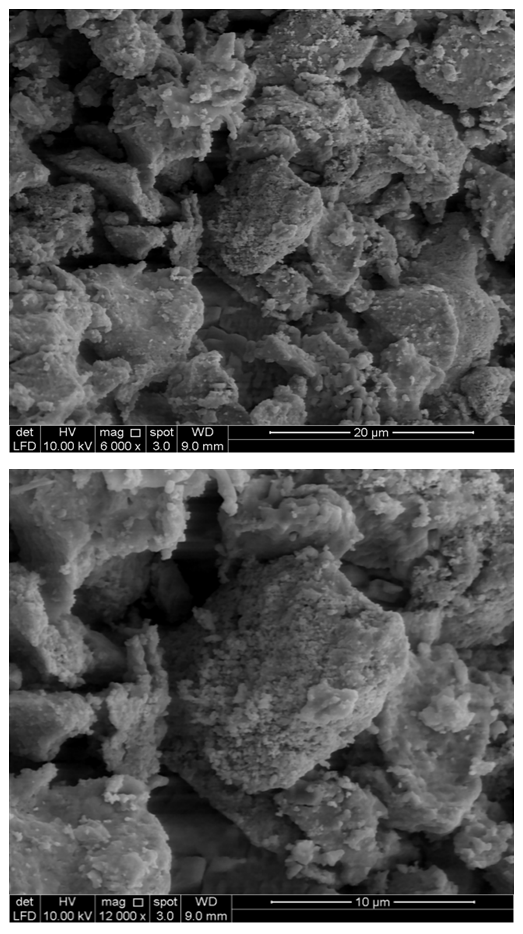 | Figure 2. SEM images of banana peels showing surface morphology |
3.2. Batch Adsorption Experiments
- Figure 3 shows the plot of adsorption capacity (qe mg/g) against the contact. Both MG and MB dyes have shown faster adsorption capacity during the first 60 min of contact between the two phases. The For qt value were 73 mg/g and 84 mg/g respectively. However, it became slower gradually until 100 mins for MG and 120 mins for MB with qt values attained 100 mg/g and 105 mg/g respectively. The reason for higher adsorption capacity at the first 100 mins of the batch experiment could be due to the higher driving force from the adsorbent, causing the dye molecules to attach to the surface of its surface easily and also the adsorption sites are not covered making it available and accessible to the dye molecules. Equilibrium was achieved at 140 mins for both dyes with MG having qe value of 107±1.3 mg/g and MB having qe value of 120±2.7 mg/g.
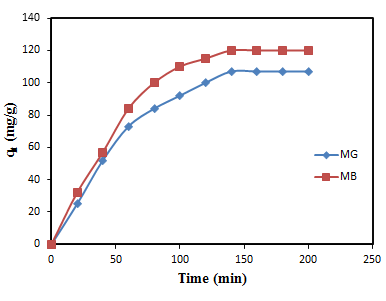 | Figure 3. Effect of contact time for the adsorption of MG and MB onto banana peel adsorbent |
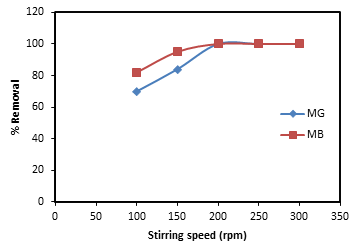 | Figure 4. Effect of stirring speed for the adsorptions of MG and MB onto banana peel |
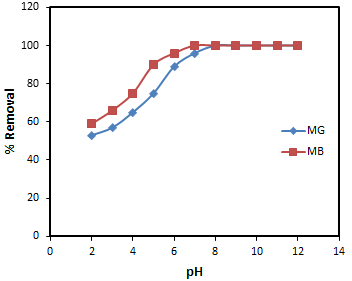 | Figure 5. Effect of pH of the solution for the adsorption of MG and MB onto banana peel |
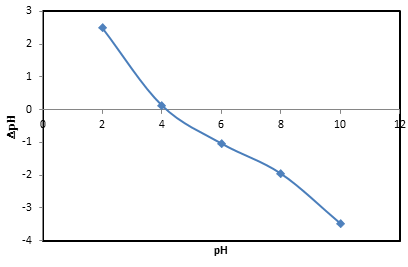 | Figure 6. Plot of point of zero charge of the adsorbent |
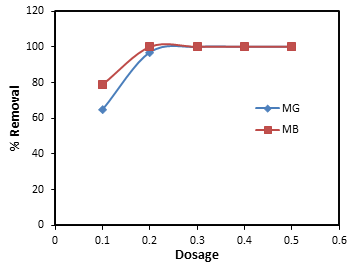 | Figure 7. Effect of adsorbent dosages for the removal of MG and MB by banana peel |
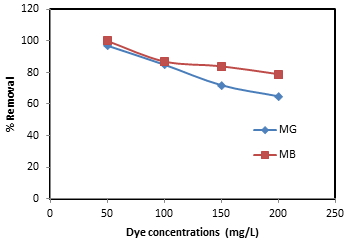 | Figure 8. Effect of various dye concentrations for the adsorption of MG and MB onto banana peel |
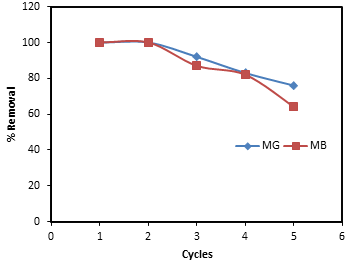 | Figure 9. Regeneration and reusability of banana peel biosorbent for the adsorption of MG and MB |
4. Conclusions
- Like other agricultural waste materials, banana peel has been proven to be good adsorbent for the removal of organic dye molecules. This could be attributed to the emergence of cellulose and lignin materials shown by the presence of various functional groups from FTIR analysis. Batch adsorption studies have shown that the biosorbent possessed high capacity of cationic MG and MB dyes. The effects of the pH of the solutions have shown that dyes removal was more favored by higher pH of the medium. The biosorbent have shown ease of application in terms of its regeneration and reusability. In general, agricultural waste banana peel can be considered as efficient and low-cost biosorbent for the effective removal of cationic dyes from water.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML