-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2017; 7(6): 232-239
doi:10.5923/j.materials.20170706.02

Ceramic Filters Impregnated with Silver Nanoparticles for Household Drinking Water Treatment
Ahmed A. Moosa, Maryam F. Muhsen
Department of Materials Engineering Technology, Engineering Technical College-Baghdad, Middle Technical University Baghdad, Iraq
Correspondence to: Ahmed A. Moosa, Department of Materials Engineering Technology, Engineering Technical College-Baghdad, Middle Technical University Baghdad, Iraq.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Silver nanoparticles (AgNPs) were synthesized from biological materials (Ziziphus plant) under microwave irradiation and later theses AgNPs were used for the reduction of microbial effect in water. Fourier Transformation infrared Spectroscopy (FTIR), UV-Vis spectrophotometer and Atomic Force Microscopy (AFM) were used to characterize AgNPs. Process parameters that affect the AgNPs synthesis, such as AgNO3 concentration, microwave exposure time, amount of extract volume and pH of the solution were also studied. The best average diameter of AgNPs (80 nm) showed by AFM. Ceramic filter and tablets were prepared from local Iraqi clay which is mixed with different combustible material (date palm seed, sawdust and charcoal). The maximum apparent porosity (50.52% porosity) was obtained at a mixing ratio of clay: charcoal of 10:4. Total Bacteria Count (TBC) was measured in the filtered water and gave a result of 0.001 CFU/ml which shows that silver nanoparticles have excellent antimicrobial activity.
Keywords: Ceramic filter, Silver Nanoparticles, Green synthesis, Ziziphus Spina Christi, AFM, Ceramic tablets
Cite this paper: Ahmed A. Moosa, Maryam F. Muhsen, Ceramic Filters Impregnated with Silver Nanoparticles for Household Drinking Water Treatment, American Journal of Materials Science, Vol. 7 No. 6, 2017, pp. 232-239. doi: 10.5923/j.materials.20170706.02.
Article Outline
1. Introduction
- Safe drinking water and sanitation are fundamental to health, survival, growth and development (Montgomery and Elimelech 2007) [1]. However, these basic necessities are still a luxury for many people worldwide. Over 1.1 billion of world citizens do not use safe drinking water, while 2.6 billion lack basic sanitation. In the developing countries, millions suffer from preventable illnesses and die every year because of the lacking of water and sanitation services (WHO: Geneva 2006) [2]. An estimated 1.8 million deaths occur each year because of a lack of access to safe water, sanitation, and proper hygiene, of which 99.8% occur in developing countries and 90% are children (Nath et al. 2006) [3]. These deaths can be reduced with proper infrastructure and education. Water is certainly the most valuable natural resource, encompass over 70% of the earth’s surface. Nevertheless, the demand for clean water is a worldwide, whether it is for human consumption, agricultural uses, or industrial uses (Cicek 2003) [4]. The United Nation Millennium Development Goals (MDG) in 2002, stressed the issue of water and sanitation on the global agenda. The MDG vision is to reduce halve the number of people without access to safe drinking water and sanitation by 50% by the year 2015 (WHO and UNICEF 2010) [5]. But up to date over half a billion people worldwide still lack access to drinking water from improved sources.Ceramic water filters manufactured with local labor using clay and a combustible organic material are one of the most promising point-of-use water-treatment technologies. The-pot-shaped filter is placed in a larger, plastic container with a spigot to also provide a safe-storage reservoir (Kallaman et al. 2011) [6]. Green synthesis of AgNPs using plant extracts is a cost effective method, environment friendly, and no toxic chemicals used (Thakkar et al. 2010) [7]. Silver nanoparticles have been synthesized using various plants. (Moosa et al., 2015) [8] used spent tea leaves extract to synthesizing AgNps which was confirmed by changing their color to dark brown due to surface plasmon resonance (SPR) phenomenon. (Moosa et al., 2015) [9] synthesized silver nanoparticles (AgNPs) using Aloe Vera plant leaves extract act as reducing and stabilizing agent.The aim of this work is to prepare silver nanoparticles (AgNPs) by green synthesis methods using Ziziphus Spina Christi leaves extract as a common Iraqi plant. Different parameters that affect the AgNPs preparation such as temperature, pH, AgNO3 and plant extract concentrations will be studied. To make ceramic water filter from Iraqi clay using different percentage of burnable materials such as (rice husk, sawdust or others) and study the porosity, water flow of these ceramic water filters. And make ceramic tablets from Iraqi clay and then impregnate these tablets with silver nanopaticles.
2. Experimental Work
2.1. Materials
- Silver nitrate (AgNO3, 99% purity, Sigma Chemical Co., Western Australia), Ziziphus Spina Christi leaves collected from local gardens, Sedimentary clays from Khan Bani Saad city, Baquba – Iraq and Charcoal taken from local market.
2.2. Preparation of Silver Nitrate Solution
- Different weights of silver nitrate (AgNO3 99% purity) were taken and add to 100 ml distilled water in 250 ml flask and then hand mixed to ensure a complete dissolution of silver nitrate. Different molarities (1, 3, 5 and 7 mM) of silver nitrate solution were prepared. The silver nitrate aqueous solution was used as a precursor to synthesis AgNPs.
2.3. Preparation of Ziziphus Spina Christi L. Leaves Extract
- Ziziphus Spina-Christi (Sidr tree or Nebka) leaves were collected from local trees in Baghdad during January month. The leaves were washed three times with distilled water to remove dust particles and other pollutants and dried at room temperature for 3-5 days. The dry leaves were ground into powder using domestic coeffee grinder.A 10 gm of Ziziphus leaves powder were boiled with 200ml distilled water in water bath at 80°C for 15 min. The leave extract aqueous solution was then filtered using filter papers (Chm, No. F1001) to obtain Ziziphus extract with a yellow color. The extract was stored in a domestic refrigerator to be used in AgNPs synthesis.
2.4. Silver Nanoparticles Synthesis
- A known volume of Ziziphus leaves plant extract was added to AgNO3 aqueous solution at different molarities and hand mixed. The mixture was allowed to react in sun-light and in the microwave for a specific time at different PH environment. The formation of AgNPs was observed by observing the change in color of the mix solution.
2.5. Effect of Plant Extract Volume on AgNPs Synthesis
- Different volume of Ziziphus leaves extract (1, 1.5, 2, 4, 6 and 8) ml were added to (99, 98.5, 98, 96, 94 and 92) ml of AgNO3 solution respectively. After microwave exposure the color was changed to brown. The best volume added to best AgNO3 concentration. The extract and AgNO3 solution mixture was then analyzed using UV, AFM and Atomic absorption. The best results were then taken at best plant extract.
2.6. Effect of Microwave Exposure Time on AgNPs
- AgNPs were synthesized using Sidr leaves extract AgNO3 aqueous solution at different concentrations (1, 3, 5 and 7) mM at different microwave exposure time (10, 20, 30, 40, 50, 60, 70, 80 and 90) sec. The solution mixture was then analyzed using UV, AFM and Atomic absorption. The best results were then taken at best exposure time.
2.7. Effect of pH on AgNPs Synthesis
- The effect of pH on preparation of AgNPs was studied under acidic, neutral and basic environment by changing the pH of the AgNO3 and leaves extract solution to (2.5, 4.5, 6.5 and 9.5). The color of reaction mixture was monitored after microwave exposure and the intensity of the SPR peaks was monitored using UV-Vis spectrometer. The best results were then took at best pH.
2.8. Effect of Combustible Materials on Ceramic Filters and Ceramic Tablets Formation
- Diffrenet types of combustible materials (sawdust, ground date palm seed and charcoal) were used with clay in ceramic filters and tablets preparation.The ceramic filters and tablets were prepared using a mixture of dry clay powder and charcoal was prepared using clay:charcoal ratios of (10:1, 10:2, 10:3, 10:4) by weight. The ceramic filters and tablets were fired at temperatures (700, 900, 1000, 1100)°C. The best results were taken at best firing temperatures.
2.9. Effect of Porosity on Ceramic Filters and Tablets
- The ceramic samples for apparent porosity measurements were prepared by mixing clay with charcoal at different ratios (10:1, 10:2, 10:3, 10:4) by weights of clay: charcoal. The green ceramic samples were then sintered the best firing temperature in the same procedures for ceramic filters and ceramic tablets in an electrical furnace with a controlled temperature in air. The best results were taken at the best porosity result.
3. Results and Discussion
3.1. FTIR for Ziziphuus Spina Christi L. Leaves Extract
- The spectra of Ziziphus spina christi L. extract (Sidr leaves extract) was obtained using a FTIR spectrophotometer (IRTracer-100, Shimadzu Co. Japan). FT-IR analysis measurements were carried out in various ranges from 4000-400 cm-1 to identify the possible biomolecules re-sponsible for the reduction of the Ag⁺ ions and the capping of AgNPs synthesized by the extract of Sidra leaves extract. FTIR spectra of the extract is shown in Figure 1. The peaks at 3360 cm-1 due to the presence of the (O-H) groups due to the presence of alcohols and phenol’s groups. The peak that appeared around 2920.23 cm-1 is related to the stretching of the C-H bonds (Mulvaney, 1996) [10]. The deep peak at 1639.49 was assigned to the stretching vibra-tion of the C=O group (Kannan et al., 2013) [11]. The peak of 1384.89 cm-1 gives an indicating to the carboxyl group.
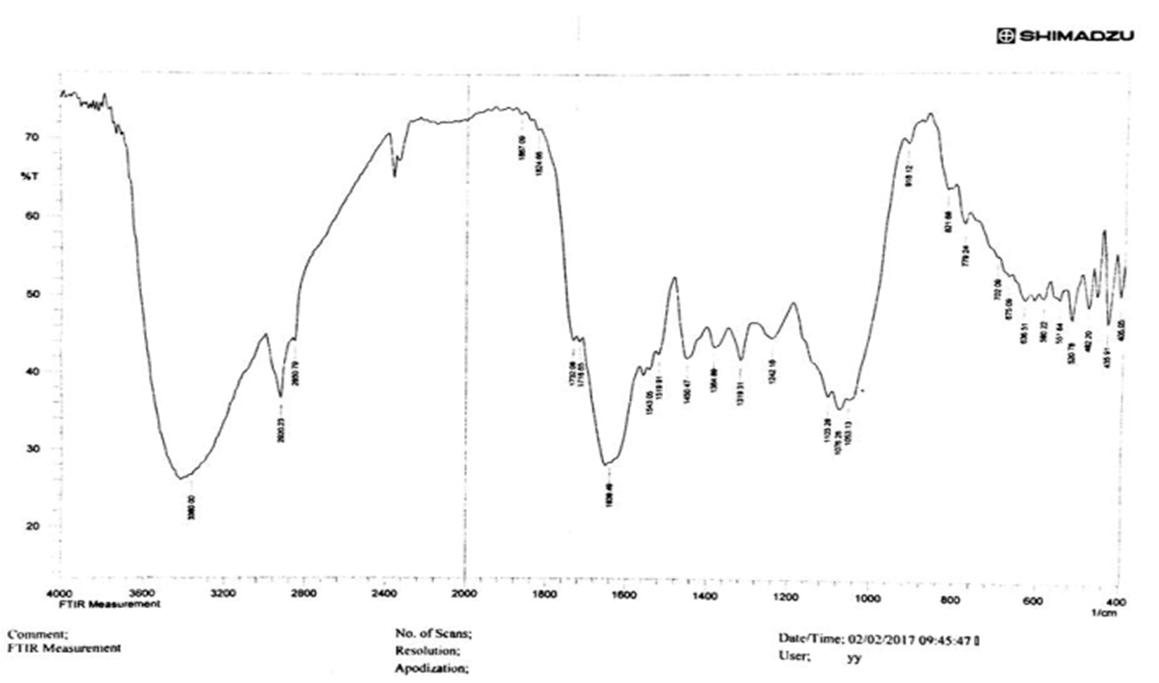 | Figure 1. FT-IR for Ziziphus (sidr) leaves extract) |
3.2. Effect of Synthesis Parameters
3.2.1. Effect of Microwave Exposure Time
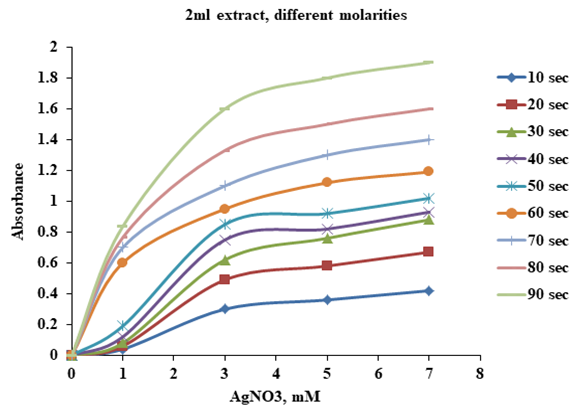
- AgNPs were synthesized using 2ml of Sidr leaves extract with 98 ml of AgNO3 aqueous solution at different concentrations (1, 3, 5 and 7) mM at different microwave exposure time (10, 20, 30, 40, 50, 60, 70, 80 and 90) sec. In Figure 2 it is clear that the absorbance increases with increasing microwave exposure time. The surface plasmon resonance (SPR) of AgNPs is slowly red shifted toward higher wavelength with increasing microwave exposure time indicating a small increase in the particle size.
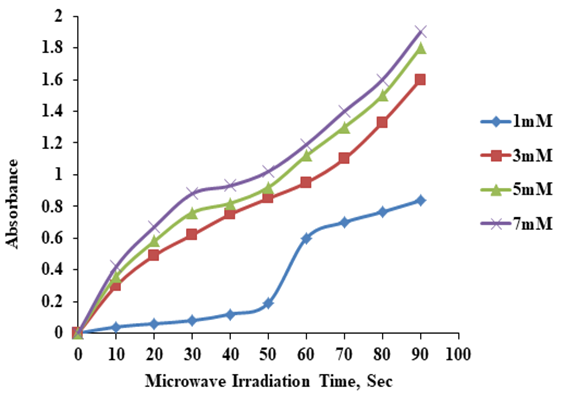 | Figure 2. UV- Visible absorbance as a function of microwave radiation time using Ziziphus leaves extract (2ml extract) |
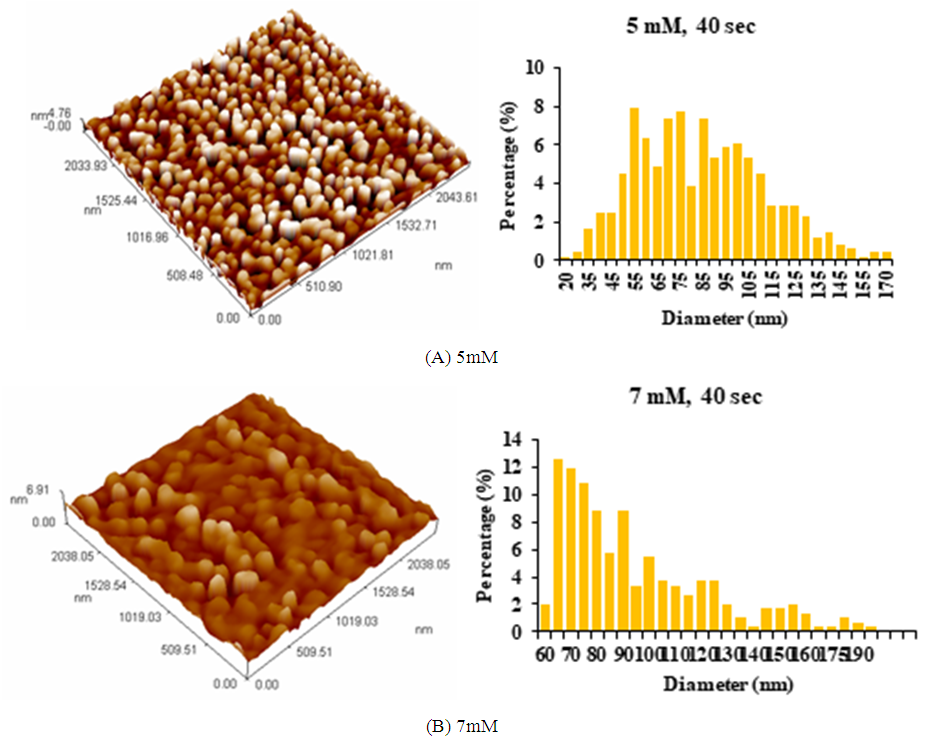 | Figure 3. AFM images of AgNPs synthesized using Ziziphus plant extracts: (A) 5mM AgNO3; (B) 7mM AgNO3, at microwave exposure time of 40 sec and 2ml extract |
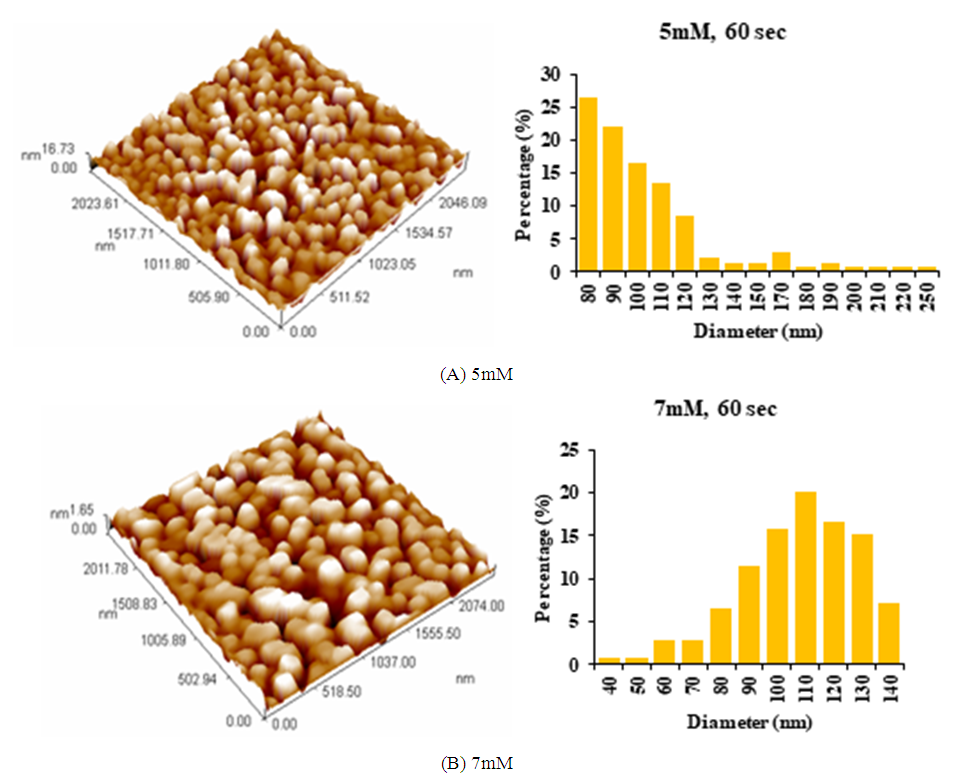 | Figure 4. AFM images of AgNPs synthesized using Ziziphus plant extracts: (A) 5mM AgNO3; (B) 7mM AgNO3, at microwave exposure time of 60 sec and 2ml extract |
3.2.2. Effect of AgNO3 Concentration (Molarity)
- The best AgNO3 concentration reaction with Ziziphus plant leaves extract for silver nanoparticles synthesis will be investigated. A 2ml of Ziziphus leaves extract was added to 98 ml of AgNO3 aqueous solution at different concentration (1, 3, 5, 7 & 9) mM with 40 sec exposure to microwave at room temperature.At AgNO3 concentration of 9 mM most of absorbance peaks are out of scale of UV–visible measurements even after the solutions were diluted 5 times with double distilled water and will not be shown.The absorbance increased with the increasing in silver nitrate concentration from 3-7 mM indicating bioreduction rate is increasing with increased concentration of AgNO3. Also the SPR peak is red shifted from 432 nm at 3mM to 454 nm at 7mM. The increase in absorbance with increasing AgNO3 concentration was also observed by (Dubey et al., 2010) [12]. A rapid increase in the absorbance is observed between 1-3 mM and small variation in the absorbance with AgNO3 concentration is observed between 3-7 mM as shown in Figure 5.
 | Figure 5. UV–Visible absorbance spectra of AgNPs suspension at different molarities (2ml Ziziphus Extract) |
3.2.3. Effect of Plant Extract Volume
- The best volume of Ziziphus leaves extract added to AgNO3 is also investigated. Different volume of Ziziphus leaves extract (1, 1.5, 2, 4, 6 and 8) ml were added to (99, 98.5, 98, 96, 94 and 92) ml of AgNO3 (5mM) solution respectively. After 40 sec of microwave exposure the color was changed to brown. It is clear that little change in the absorbance was observed at microwave exposure time of 40 sec. Figure 6 shows that rapid increase in the absorbance was observed for the extract volume 0-2 ml and little variation in the absorbance between 2-6 ml. The best extract volume was selected to be 2 ml.
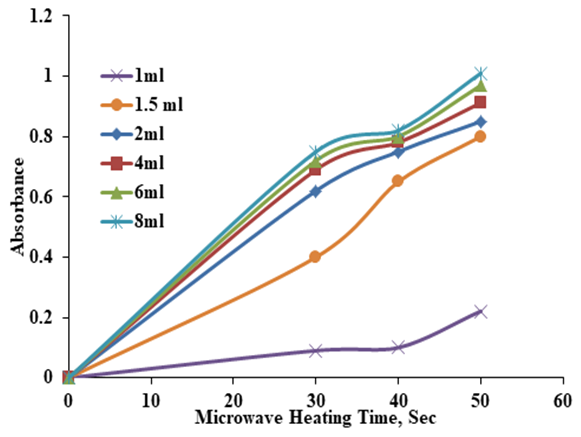 | Figure 6. UV- Visible absorbance spectra of AgNPs Vs. microwave exposure time at different Ziziphus leaves extract volume, 5mM |
3.2.4. Effect of pH on Synthesis of AgNPs
- The effect of pH on preparation of AgNPs was studied under acidic, neutral and basic environment by changing the pH of the AgNO3 and leaves extract solution to (2.5,4.5, 6.5 and 9.5) using 5mM of AgNO3 and 2ml extract volume with a 40 sec exposure time in microwave as shown in Figure 7.
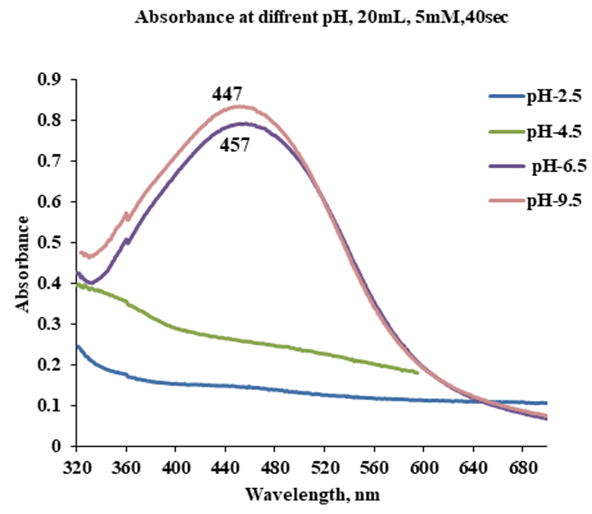 | Figure 7. UV–Visible absorbance spectra of AgNPs suspension at different pH values (2ml Ziziphus Extract, 5mM AgNO3, 40 sec) |
3.3. Preparation of Ceramic Filters and Ceramic Tablets
3.3.1. Effect of Combustible Materials
- Many types of combustible materials were used. The use of hand molded clay with sawdust resulted in unsound ceramic body. This is due to the cellulose that found in sawdust which leads to break the bond by absorbing the water in the clay and lead to cracks in the ceramic body. This problem could be avoided by using a hydraulic press to form the ceramic filters and tablets. The second material used was ground date palm seed with clay. After firing, the ceramic body obtained was very brittle and easy to break. This is due to the fine particle size of ground date palm seed, which leaves a large number of fine interconnected pores after firing. The third material used was clay with ground charcoal. After firing it was found that a sound porous ceramic body was obtained. In this work the best combustible material was charcoal.
3.3.2. Effect of Firing Temperature
- The ceramic filters and tablets were fired at temperatures (700, 900, 1000, 1100)°C. It was found that at 700°C not all the combustible material (charcoal) in the clay was completely burned and the ceramic body still contains carbonized materials and resulted in unsound ceramic body. At 900 and 1000°C the strength of ceramic filters and tablets are very good they are porous and all charcoal was completely burned. At 1000°C the hardness and stability of ceramics were better than 900°C. At 1100°C the ceramic filters and tablets are brittle and easy to break and that was attributed to clay type. Red clays afford temperatures up to maximum 1050°C after that they become very brittle and easy to break. In this work the best firing temperature was 1000°C.
3.3.3. Effect of Porosity
- The apparent porosity (% AP) is the open pore volume as a percentage of the bulk volume. The ceramic samples for apparent porosity measurements were prepared by mixing clay with charcoal at different ratios (10:1, 10:2, 10:3, 10:4) by weights of clay: charcoal. The green ceramic samples were then sintered at 1000°C in the same procedures for ceramic filters and ceramic tablets in an electrical furnace with a controlled temperature in air. A dummy clay sample without charcoal is also prepared and sintered in the same procedures for apparent density comparison. The porosity of clay without any additives is equal to (27.33 %) and the porosity for ceramic filters and tablets in the ratios (10:1, 10:2, 10:3 and 10:4) was (42.84%, 45.09%, 48.95% and 50.52%) respectively. It is clear from Figure 8. The porosity increases with increasing charcoal amount. Depending on the apparent porosity amount. In this work, the ratio 10:4 was chosen as the best amount to obtained a best apparent porosity.
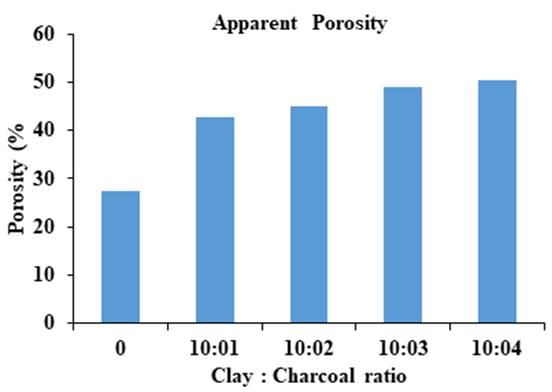 | Figure 8. Apparent porosity in ceramic filters and tablets |
3.4. Impregnation of Ceramic Tablets with Silver Nanoparticles
- The ceramic tablets with clay: charcoal ratio of 10:4 were impregnated with the prepared AgNPs solution. The AgNPs was prepared using the best condition in this work 2ml Ziziphus leaves extract with 5mM AgNO3 solution (neutral pH) in the microwave for 40 sec. A 0.22 g/L of AgNPs solution will be taken depending on previous researches with an average particles size of 82nm was mixed with 1 litter of distelled water. The ceramic tablets were immersed in this solution for 24 hrs. The ceramic tablets were then dried in an oven for 4 hrs at 50°C. The ceramic tablets were then put inside the ceramic filters and water was added to be filtered. About 1 liter of water per hour will be filtered. Filtration system shown in Figure 9.
 | Figure 9. Filtration system, (a) Ceramic Filter with ceramic Tablets; Ceramic tablets |
4. Conclusions
- The green synthesis of AgNPs method provides a simple, efficient, environmental ecofriendly and cost effective route using Ziziphus Spina Christi L. leaves extract with silver nitrate solution under microwave irradiation for 40 sec. The best synthesis parameters for AgNPs are 5mM of silver nitrate and 2ml of Ziziphus leaves extract. The best pH for synthesis of AgNPs was the neutral and alkaline, because acidic conditions are not suitable for promoting biosynthesis of silver nanoparticles. Ceramic filter and ceramic tablets were prepared by mixing local Iraqi clays with charcoal by hand molding. Charcoal achieved best combination with clay in hand molding process. Ceramic filter and ceramic tablets were fired after molding and drying at 1000°C because at this temperature gives better ceramics properties. Ceramic filter and ceramic tablets gave a better flow rate of (1) litter/ hr at clay to charcoal ratio of 10:4 which give good apparent porosity percent of (50.52%). The antimicrobial properties of silver nanoparticles were very efficient when it was impregnated into ceramic tablets.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML