| [1] | de'Gennaro, M., Cappelletti, P., Langella, A., Perrotta, A. and Scarpati, C.: Genesis of zeolites in the Neapolitan Yellow Tuff: geological, volcanological and mineralogical evidence. Contributions to Mineralogy and Petrology 139 (2000) 17 – 35. |
| [2] | Harpel C.J., Kyle, P.R. and Dunbar, N.W.: Englacial tephrostratigraphy of Erebus volcano, Antarctica. Journal of Volcanology and Geothermal Research 177 (2008) 549 – 568. |
| [3] | Weckhuysen, B.M., and Yu, J.: Recent advances in zeolite chemistry and catalysis. Chemical Society Reviews 44 (2015) 7022 – 7024. |
| [4] | Coombs, D.S., Alberti, A., Armbruster, T., Artioli, G., Colella, C., Galli, E., Grice, J.D., Liebau, F., Mandarino, J.A., Minato, H., Nickel, E.H., Passaglia, E., Peacor, D.R., Quartieri, S., Rinaldi, R., Ross, M., Sheppard R.A., Tillmanns, E., Vezzalini, G.: Recommended Nomenclature for Zeolite Minerals: Report of The Subcommittee on Zeolites of the International Mineralogical Association, Commission on New Minerals and Mineral Names. The Canadian Mineralogist 35 (1997) 1571 – 1606. |
| [5] | Byrappa, K. and Yoshimura, M. 2001. Hydrothermal Synthesis and Growth of Zeolites (ADVANCED STRUCTURE STUDIES) in Handbook of Hydrothermal Technology. pp 315 – 414. |
| [6] | Iijima, A.: Geology of natural zeolites and zeolitic rocks. Pure & Applied Chemistry 52 (1980) 2115 – 2130. |
| [7] | M Fischer, M.: Structure and bonding of water molecules in zeolite hosts: Benchmarking plane-wave DFT against crystal structure data. Zeitschrift für Kristallographie-Crystalline Materials 230 (2015) 325 – 336. |
| [8] | Hashimoto, S.: Zeolite photochemistry: impact of zeolites on photochemistry and feedback from photochemistry to zeolite science. Journal of Photochemistry and Photobiology C: Photochemistry Reviews 4 (2003) 19 – 49. |
| [9] | Granda Valdés, M., Pérez-Cordoves, A.I. and Díaz-García, M.E.: Zeolites and zeolite – based materials in analytical chemistry. Trends in Analytical Chemistry 25 (2006) 24 – 30. |
| [10] | Armbruster, T. and Gunter, M.E. 2001. Crystal structure of natural zeolites. In Bish, D.L. Ming, D.W. (Eds). Natural Zeolites: occurrence, properties, applications. Mineralogical Society of America, Geochemical Society 45: 1 – 68. |
| [11] | Farkaš, A., Rožić M. and Barbarić-Mikočević, Ž.: Ammonium exchange in leakage waters of waste dumps using natural zeolite from the Krapina region, Croatia. Journal of Hazardous Materials B 117 (2005) 25–33. |
| [12] | Widiastuti, N., Wu, H. Ang, H.M. and Zhang D.: Removal of ammonium from greywater using natural zeolite. Desalination 277 (2011) 15 – 23. |
| [13] | Sponer, J.E., Sobalik, Z., Leszczynski, J. and Wicthterlova, B.: Effect of metal coordination on the charge distribution over the cation binding sites of zeolites. A combined experimental and theoretical study. Journal of Physical Chemistry, B 105 (2001) 8285 – 8290. |
| [14] | Wang, J. 2006. Thermodynamics of dehydration and hydration in natrolite and analcime. A Thesis Presented to The Graduate School of The University of Florida in Partial Fulfillment of The Requirements for The Degree of Master of Science. University of Florida. In http://ufdc.ufl.edu/UFE0015761/00001/2j.html accessed on 18th March 2016. |
| [15] | Khodayar, M. and Franzson, H.: Fracture pattern of Thjórsárdalur central volcano with respect to rift – jump and a migrating transform zone in South Iceland. Journal of Structural Geology 29 (2007) 898 – 912. |
| [16] | Murata, K.J., Firmoso, M.L.L. and Roisenberg, A.: Distribution of zeolites in lavas of southeastern Parana Basin, state of Rio Grande dolSul, Brazil. The Journal of Geology 95 (1987) 455 – 467. |
| [17] | Weisenberger, T. and Selbekk, R.S.: Multi – stage zeolite facies mineralization in the Hvalfjördur area, Iceland. International Journal of Earth Sciences. (GeolRundsch) 98 (2009) 985 – 999. |
| [18] | Morris, H.H., Whyte Jr., J.R., Forbus, E., Dentan, C.M. and Collins, D.R. 1985. Method of Treating Zeolite Ores to Remove Discoloring Impurities and Improve its Brightness and Resulting Finely Ground Zeolite. US Patent Number 4510254. Issue Date: Apr9, 1985. In https://0x9.me/xJHuD. Accessed on 27Apr. 2012. |
| [19] | Weisenberger, T. 2009. Zeolite in fissures of crystalline basement rocks. PhD thesis, University of Freiburg. Freiburg im Breisgau, Germany. In http://www.freidok.uni–freiburg.de/volltexte/6757/pdf/Dissertation_Tobias_Weisenberger.pdf. Accessed on 08 Aug. 2012. |
| [20] | Liou, J.G., Maruyama, S. and Cho, M. Phase equilibria and mineral parageneses of metabasites in low – grade metamorphism. Mineralogical Magazine 49 (1985): 321 – 333. |
| [21] | Thompson, A.B.: P CO2 in low-grade metamorphism; zeolite, carbonate, clay mineral, prehnite relations in the system CaO-Al2O3-SiO2-CO2-H2O. Contributions to Mineralogy and Petrology 3 (1971)145 – 161. |
| [22] | Pe-piper, G.: Mode of Occurrence, Chemical Variation and Genesis of Mordenite and Associated Zeolites from the Morden Area, Nova Scotia, Canada. The Canadian Mineralogist 38 (2000) 1215 – 1232. |
| [23] | Hay, R.L. Geologic occurrence of zeolites and some associated minerals. Pure & Applied Chemistry 58 (1986) 1339 – 1342. |
| [24] | U.S. Geological Survey, Mineral Commodity Summaries. January 2011. Zeolites (Natural). In http://minerals.usgs.gov/minerals/pubs/commodity/zeolites/mcs-2011-zeoli.pdf. Accessed on 08 Aug. 2012. |
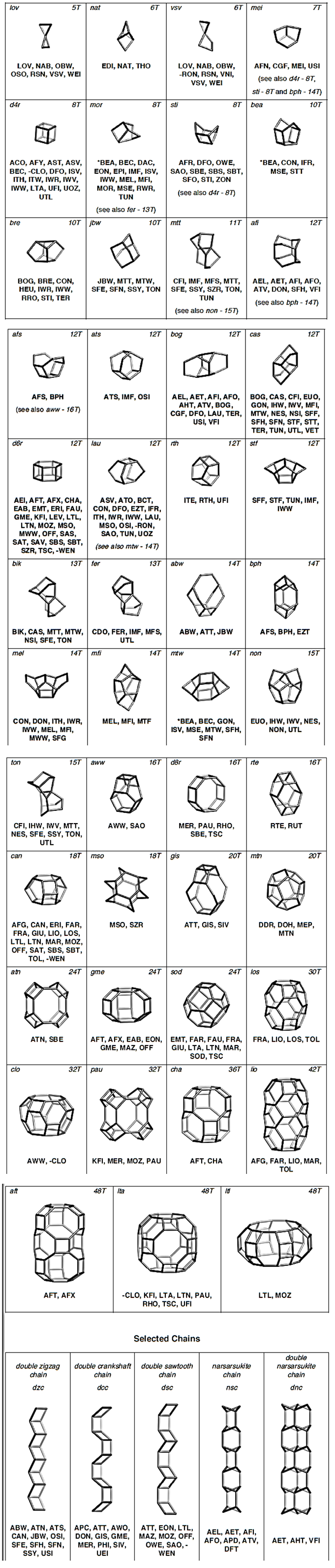
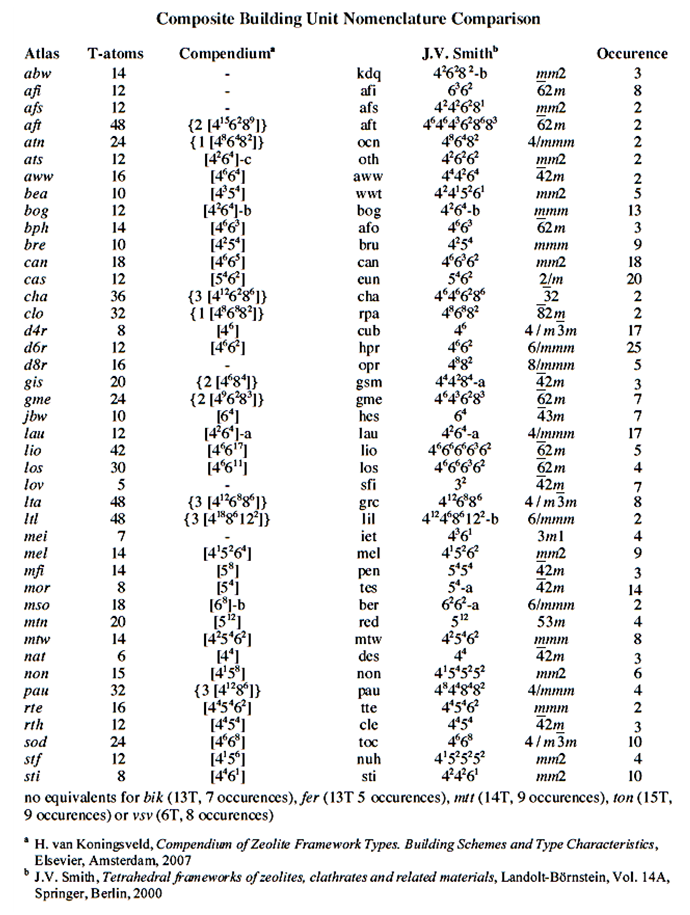
| [25] | Baerlocher, Ch., McCusker, L. and Olson, D.H. 2007. Atlas of Zeolite Framework Types, 6th ed. Elsevier Publishing co. Amsterdam. |
| [26] | Eleroğlu, H. and Yalçın, H.: Use of natural zeolite-supplemented litter increased broiler production. South African Journal of Animal Science 35 (2005) 90 – 97. |
| [27] | Boettinger, J.L., and Ming, D.W. 2002. Zeolites. In: J.B. Dixon and D.G. Schulze (Eds.) Soil Mineralogy with Environmental Applications. Soil Science Society of America Book Series, no. 7, Madison, WI. pp 585 – 610. |
| [28] | Smical, I.: Properties of natural zeolites in benefit of nutrition and health. HVM Bioflux 3 (2011) 51 – 57. |
| [29] | Ratner, M.A. and Ratner, D. 2003.: Nanotechnology: A Gentle Introduction to the Next Big Idea. Prentice Hall Professional. E-book in https://0x9.me/9so5D. Accessed on 24 October 2015. |
| [30] | Estermann, M., McCusker, L.B., Baerlocher, C., Merrouche, A. and Kessler, H.: A Synthetic Gallophosphate Molecular Sieve with a 20-Tetrahedral-Atom Pore Opening. Nature 352 (1991) 320 – 323. |
| [31] | McCusker, L.B., Baerlocher, C., Jahn, E. and Bulow, M.: The Triple Helix Inside the Large – Pore Aluminophosphate Molecular Sieve VPI – 5. Zeolites 11 (1991) 308 – 313. |
| [32] | Wessels, T., Baerlocher, C., McCusker, L.B. and Creyghton, E.J.: An Ordered Form of the Extra – Large – Pore Zeolite UTD – 1: Synthesis and Structure Analysis from Powder Diffraction Data. Journal of the American Chemical Society 121 (1999) 6242 – 6247. |
| [33] | Yoshikawa, M., Wagner, P., Lovallo, M., Tsuji, K., Takewaki, T., Chen, C.Y., Beck, L., Jones, C., Tsapatsis, M., Zones, S.I. and Davis. M.E. Synthesis, Characterization, and Structure Solution of CIT – 5, a New, High – Silica, Extra – Large – Pore Molecular Sieve,” The Journal of Physical Chemistry B 102 (1998) 7139 – 7147. |
| [34] | Jiang, J., Yun, Y., Zou, X., Jorda, J.L. and Corma, A.: ITQ-54: a multi-dimensional extra-large pore zeolite with 20 × 14 × 12-ring channels. Chemical Science 6 (2015) 480 – 485. |
| [35] | McCusker, L.B., Grosse–Kunstleve, R.W., Baerlocher, C., Yoshikawa, M. and Davis, M.E.: Synthesis optimization and structure analysis of the zincosilicate molecular sieve VPI – 9. Microporous Materials 6 (1996) 295 – 309. |
| [36] | Robrig, C. and Gies, H.: A new zincosilicate zeolite with nine-ring channels. Angewandte Chemie International Edition 34 (1995) 63 – 65. |
| [37] | Robrig, C., Gies, H. and Marler, B.: Rietveld refinement of the crystal structure of the synthetic porous zincosilicate VPI – 7. Zeolites 14 (1994) 498 – 503. |
| [38] | Nguyen, S.T. 2012. Multiple Gas Sensing Device Based on Nano – Porous Structure of Zeolite Coated With Nile Red Dye. A Dissertation Submitted to the Temple University Graduate Board In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy. In https://0x9.me/cCwv7. Accessed on 18 March 2016. |
| [39] | Lobo, R.F.: Introduction to the structural chemistry of Zeolites. In Auerbach, S.M., Carrado, K.A. & Dutta, P.K. 2003. Handbook of Zeolite Science and Technology, CRC Press, pp 80-112. E-book in https://0x9.me/1Uv2z. Accessed on 24 October 2015. |
| [40] | Bedioui, F.: Zeolite-encapsulated and clay-intercalated metal porphyrin, phthalocyanine and Schiff-base complexes as models for biomimetic oxidation catalysts: an overview. Coordination Chemistry Reviews 144 (1995) 39 – 68. |
| [41] | Qinhua, X. and Aizhen, Y.: Hydrothermal Synthesis and Crystallization of Zeolites. In: Byrappa, K. (Ed.), Hydrothermal Growth of Crystals. Progress in Crystal Growth and Characterization of Materials 21 (1990) 29 – 70. |
| [42] | Weitkamp, J.: Zeolites and catalysis. Solid State Ionics 131 (2000) 175 – 188. |
| [43] | Castaldi, P., Santona, L., Enzo, S. and Melis, P.: Sorption processes and XRD analysis of a natural zeolite exchanged with Pb2+, Cd2+ and Zn2+ cations. Journal of Hazardous Materials 156 (2008) 428 – 434. |
| [44] | Rouquerol, J., Avnir, D., Fairbridge, C. W., Everett, D. H., Haynes, J. H., Pernicone, N., Ramsay, J. D. F., Sing, K.S.W. and Unger, K.K.: Recommendations for the characterization of porous solids (Technical Report). Pure and Applied Chemistry 66 (1994) 1739 – 1758. |
| [45] | Bento Ribeiro, L.E., Alcântara, G.P., Gonçalves Andrade, C.M., Fruett, F.: Analysis of the Planar Electrode Morphology Applied to Zeolite Based Chemical Sensors. Sensors & Transducers 193 (2015) 80 – 85. |
| [46] | Kosinov, N., Gascon, J., Kapteijn F. and Hensen, E.J.M.: Recent developments in zeolite membranes for gas separation. Journal of Membrane Science 499 (2016) 65 – 79. |
| [47] | Ackley, M.W., Rege, S.U. and Saxena, H.: Application of natural zeolites in the purification and separation of gases. Microporous and Mesoporous Materials 61 (2003) 25 – 42. |
| [48] | Roque-Malherbe, R.: Complementary approach to the volume filling theory of adsorption in zeolites. Microporous and Mesoporous Materials 41 (2000) 227 – 240. |
| [49] | Li, C., Zhong, H., Wang, S., Xue, J. and Zhang, Z.: A novel conversion process for waste residue: Synthesis of zeolite from electrolytic manganese residue and its application to the removal of heavy metals. Colloids and Surfaces A: Physicochemical and Engineering Aspects 470 (2015) 258 – 267. |
| [50] | Visa, M.: Synthesis and characterization of new zeolite materials obtained from fly ash for heavy metals removal in advanced wastewater treatment. Powder Technology 294 (2016) 338 – 347. |
| [51] | Clement, R.E., Eiceman, G.A. and Koester, C.J.: Environmental – analysis. Analytical Chemistry 67 (1995) R221 – R255. |
| [52] | Hui K.S., Chao, C.Y.H. and Kot, S.C.: Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. Journal of Hazardous Materials B 127 (2005) 89 – 101. |
| [53] | Maretto, M. Vignola, R., Williams, C.D., Bagatin, R., Latini, A. and Papini M.P.: Adsorption of hydrocarbons from industrial wastewater onto a silica mesoporous material: Structural and thermal study. Microporous and Mesoporous Materials 203 (2015) 139 – 150. |
| [54] | Eriksson, E., Auffarth, K., Henze, M. and Ledin, A.: Characteristics of grey wastewater, Urban Water 4 (2002) 85 – 104. |
| [55] | Belanger, S.E., Bowling, J.W., Lee, D.M., LeBlanc, E.M., Kerr, K.M., McAvoy, D.C., Christman, S.C. and Davidson, D.H.: Integration of aquatic fate and ecological responses to linear alkyl benzene sulfonate (LAS) in model stream ecosystems, Ecotoxicology and Environmental Safety 52 (2002) 150 – 171. |
| [56] | Mumpton, F.A. 1999.: La rocamagica: Uses of natural zeolites in agriculture and industry. Proceedings of the National Academy of Sciences of the United States of America 96 pp. 3463 – 3470. |
| [57] | Filippidis, A.A.: Environmental, industrial and agricultural applications of Hellenic Natural Zeolite. Hellenic Journal of Geosciences 45 (2010) 91 – 100. |
| [58] | Kosobucki, P., Kruk, M. and Buszewski, B.: Immobilization of selected heavy metals in sewage sludge by natural zeolites. Bioresource Technology 99 (2008) 5972 – 5976. |
| [59] | Kalló, D.: Applications of Natural Zeolites in Water and Wastewater Treatment. Reviews in Mineralogy and Geochemistry 45 (2001) 519 – 550. |
| [60] | Çulfaz, M. and Yağız, M.: Ion exchange properties of natural clinoptilolite: Lead-Sodium and Cadmium-Sodium equilibria. Separation and Purification Technology 37 (2004) 93 – 105. |
| [61] | Ming, D.W. and Dixon, J.B.: Quantitative Determination of Clinoptilolite in Soils by a Cation – Exchange Capacity Method. Clays and Clay Minerals 35 (1987) 463 – 468. |
| [62] | Pabalan, R. T. and Bertetti, F. P. 2001. Cation exchange capacity of natural zeolites. In: Bish, D. and Ming, D. (Eds) Natural zeolites: occurrence, properties, applications. Reviews in Mineralogy and Geochemistry. Mineralogical Society of America. 453 – 518. |
| [63] | Tsitsishvili G. V., Andronikashvili T. G., Kirov G. N., Filizova, L.D. 1992. Natural Zeolites. Ellis Horwood, New York. pp 295. |
| [64] | Perego, C., Bagatin, R., Tagliabue, M. and Vignola, R.: Zeolites and Related Mesoporous Materials for Multi – talented Environmental Solutions. Microporous and Mesoporous Materials 166 (2013) 37 – 49. |
| [65] | Demir, A., Gunay, A. and Debik, E.: Ammonium removal from aqueous solution by ion exchange using packed bed natural zeolite. Water SA 28 (2002) 329 – 335. |
| [66] | Du, Q., Liu, S., Cao, Z. and Wang, Y.: Ammonia removal from aqueous solution using natural Chinese clinoptilolite. Separation and Purification Technology 44 (2005) 229 – 234. |
| [67] | Ates, A., Reitzmann, A., Hardacre, C. and Yalcin, H.: Abatement of nitrous oxide over natural and iron modified natural zeolites. Applied Catalysis A: General 407 (2011) 67 – 75. |
| [68] | Mertens, G., Snellings, R., Van Balen, K., Bicer-Simsir, B., Verlooy, P. and Elsen, J.: Pozzolanic reactions of common natural zeolites with lime and parameters affecting their reactivity. Cement and Concrete Research 39 (2009) 233 – 240. |
| [69] | Ibrahim, S.A.B. 2007.: Synthesis and Characterization of Zeolites from Sodium Aluminosilicate Solution. Thesis submitted in fulfilment of the requirements for the degree of Master of Science. In http://eprints.uthm.edu.my/6782/1/SITI_AIDA_IBRAHIM.pdf Accessed on 18 July 2012. |
| [70] | Ho, Y.S.: Review of second-order models for adsorption systems. Journal of Hazardous Materials B 136 (2006) 681 – 689. |
| [71] | Ho, Y.S. and McKay, G.: Pseudo-second order model for sorption processes. Process Biochemistry 34 (1999) 451 – 465. |
| [72] | Dursun, G., Cicek, H. and Dursun, A.Y.: Adsorption of phenol from aqueous solution by using carbonised beet pulp. Journal of Hazardous Materials 125 (2005) 175 – 182. |
| [73] | Mall, I.D., Srivastava, V.C. and Agarwal, N.K.: Removal of orange – G and methyl violet dyes by adsorption onto bagasse fly ash – kinetic study and equilibrium isotherm analysis. Dyes and Pigments 69 (2006) 210 – 223. |
| [74] | Wang, S., Li, L. Wu, H. and Zhu, Z.H.: Unburned carbon as low-cost adsorbent for treatment of methylene blue-containing wastewater. Journal of Colloid and Interface Science 292 (2005) 336 – 343. |
| [75] | Sarioglu, M.: Removal of ammonium from municipal wastewater using natural Turkish (Dogantepe) zeolite. Separation and Purification Technology 41 (2005) 1 – 11. |
| [76] | Englert, A.H. and Rubio, J.: Characterization and environmental application of a Chilean natural zeolite. International Journal of Mineral Processing 75 (2005) 21 – 29. |
| [77] | Jorgensen, T.C. and Weatherley, L.R.: Ammonia removal from wastewater by ion exchange in the presence of organic contaminants. Water Research 37 (2003) 1723 – 1728. |
| [78] | Zita, A. and Hermansson, M.: Effects of Bacterial Cell Surface Structures and Hydrophobicityon Attachment to Activated Sludge Flocs. Applied and Environmental Microbiology 63 (1996) 1168–1170. |
| [79] | Kalló, D. 1993.: Wastewater purification in Hungary using natural zeolites: In Ming D.W. and Mumpton, F.A. (Eds). 1993. Natural Zeolites. Int. Comm. Natural Zeolites, Brockport, New York, pp. 341 – 350. |
| [80] | Kubota, M., Nakabayashi, T., Matsumoto, Y., Shiomi, T., Yamada, Y., Ino, K., Yamanokuchi, H., Matsui, M., Tsunoda, T., Mizukami, F. and Sakaguchi, K.: Selective adsorption of bacterial cells onto zeolites. Colloids and Surfaces B: Biointerfaces 64 (2008) 88 – 97. |
| [81] | Poortinga A.T., Bos, R., Norde, W. and Busscher, H.J.: Electric double layer interactions in bacterial adhesion to surfaces.Surface Science Reports 47 (2002) 1 – 32. |
| [82] | Schneider, C. 2010.: The Surface Charge of Soft and Hard Sphere Colloidal Particles – Experimental Investigation and Comparison to Theory. Dissertation. Zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften (Dr.rer. nat.) im Fach Chemie der Fakultät für Biologie, Chemie und Geowissenschaften der Universität Bayreuth. In https://0x9.me/lo9h8. Accessed on 18 July 2015. |
| [83] | Kithome, M., Paul, J.W., Lavkulich, L.M. and Bomke, A.A.: Effect of pH on ammonium adsorption by natural Zeolite clinoptilolite. Communications in Soil Science and Plant Analysis 30 (1999) 1417 – 1430. |
| [84] | Rožić, M., Cerjan-Stefanović, Š.,Kurajica, S., Vančina, V. and Hodžić, E.: Ammoniacal nitrogen removal from water by treatment with clays and zeolites. Water Research 34 (2000) 3675 – 3681. |
| [85] | Cardoso, A.M., Horn, M.B., Ferret, L.S., Azevedo, C.M.N. and Pires, M.: Integrated synthesis of zeolites 4A and Na–P1 using coal fly ash for application in the formulation of detergents and swine wastewater treatment. Journal of Hazardous Materials 287 (2015) 69 – 77. |
| [86] | Pavelić, K., Hadžija, M., Bedrica, L., Pavelic, J., Dikic, I., Katic, M., Kralj, M., Bosnar, M.H., Sanja, K., Poljak-Blaži, M., Križanac S., Stojkovic, R., Jurin, M., Subotic, B. and Colic, M.: Natural zeolite clinoptilolite: new adjuvant in anticancer therapy. Journal of Molecular Medicine 78 (2001) 708 – 720. |
| [87] | Moore S.M., Rintoul R.C., Walker R., Chilvers E.R., Haslett C. and Sethi T.: The presence of a constitutively active phosphoinositide 3-kinase in small cell lung cancer cell mediates anchorage-independent proliferation via a protein kinase B and p70s6k- dependent pathway. The Journal of Cancer Research 58 (1998) 5239 – 5247. |
| [88] | Pavelic, K.: Natural zeolite clinoptilolite: new adjuvant in anticancer therapy. Journal of Molecular Medicine 78 (2001) 708 – 720. |
| [89] | Heinemann, H.: Technological Applications of Zeolites in Catalysis. Catalysis Reviews: Science and Engineering 23 (1981) 315 – 328 |
| [90] | Davis, M.E.: Zeolite-based catalysts for chemicals synthesis. Microporous and Mesoporous Materials 21 (1998) 173 – 182. |
| [91] | Venuto, P.B.: Organic catalysis over zeolites: A perspective on reaction paths within micropores. Microporous Materials 2 (1994) 297 – 411. |
| [92] | Van Bokhoven Group Research, 2007. Structure-selectivity relationship in zeolite-catalyzed alkylation reactions. In http://www.vanbokhoven.ethz.ch/research/ndan. Accessed on 31 July 2013. |
| [93] | Farneth, W.E. and Gorte, R.J.: Methods for Characterizing Zeolite Acidity. Chemical Reviews 95 (1995) 615 – 635. |
| [94] | Bronsted, J.N.: The Acid-Basic Function of Molecules and its Dependency on the Electric Charge Type. Journal of Physical Chemistry 30 (1926) 777–790. |
| [95] | Zhu, X., Liu, S., Song, Y. and Xu. L.: Catalytic cracking of C4 alkenes to propene and ethene: Influences of zeolites pore structures and Si/Al2 ratios. Applied Catalysis A: General 288 (2005) 134 – 142. |
| [96] | Derouane, E.G.: Zeolites as solid solvents. Journal of Molecular Catalysis A: Chemical 134 (1998) 29 – 45. |
| [97] | Guisnet, M. and Magnoux, P.: Coking and deactivation of zeolites: Influence of the Pore Structure. Applied Catalysis 54 (1989) 1 – 27. |
| [98] | Ribeiro, F.R., Alvarez, F., Henriques, C., Lemos, F., Lopes, J.M. and Ribeiro, M.F.: Structure-activity relationship in zeolites. Journal of Molecular Catalysis A: Chemical 96 (1995) 245 – 270. |
| [99] | Weisz, P.B. and Frilette, V.J.: Intracrystalline and Molecular-Shape-Selective Catalysis by Zeolite Salts. The Journal of Physical Chemistry 64 (1960) 382 – 382. |
| [100] | Csicsery S.M. 1976. In J.A. Rabo (Ed.), Zeolite Chemistry and Catalysis, ACS Monograph 171, American Chemical Society, Washington, DC. p. 680. |
| [101] | Stöcker, M.: Gas phase catalysis by zeolites. Microporous and Mesoporous Materials 82 (2005) 257 – 292 |
| [102] | Fritz, A. and Pitchon, B.: The current state of research on automotive lean NOx catalysis. Applied Catalysis B: Environmental 13 (1997) 1 – 25. |
| [103] | Shin, H.K., H. Hirabayashi, H. Yahiro, M. Watanabe and Iwamoto, M..: Selective catalytic reduction of no by ethene in excess oxygen over platinum ion-exchanged MFI zeolites. Catalysis Today 26 (1995) 13 – 21. |
| [104] | Junaid, A.S.M Yin, H., Koenig, A., Swenson, P., Chowdhury, J., Burland, G., McCaffrey, W.C. and Kuznicki, S.M.: Natural zeolite catalyzed cracking-assisted light hydrocarbon extraction of bitumen from Athabasca oil sands. Applied Catalysis A: General 354 (2009) 44 – 49. |
| [105] | Montalvo, S., Guerrero, L., Borja, R., Sánchez, E., Milán, Z., Cortés, I. and Angeles de la laRubia, M.: Application of natural zeolites in anaerobic digestion processes: A review. Applied Clay Science 58 (2012) 125 – 133. |
| [106] | Levenets, V.V., Lonin, A.Y., Omelnik O.P. and Shchur A.O.: PIXE in the studies of stable cesium sorption from water solutions. X-Ray Spectrometry 44 (2015) 447 – 450. |
| [107] | Lonin, A.Y., Levenets, V.V., Neklyudov, I.M. and Shchur, A.O.: The usage of zeolites for dynamic sorption of cesium from wastewaters of nuclear power plants. Journal of Radioanalytical and Nuclear Chemistry 303 (2015) 831 – 836. |
| [108] | Abdel Moamen, O.A., Ismail, I.M., Abdelmonem, N. and Abdel Rahmana R.O.: Factorial design analysis for optimizing the removal of cesium and strontium ions on synthetic nano-sized zeolite. Journal of the Taiwan Institute of Chemical Engineers 55 (2015) 133 – 144. |
| [109] | Ayar, N., Keçeli, G., Kurtoğlu, A. E. and Atun, G.: Cationic dye adsorption onto natural and synthetic zeolites in the presence of Cs+ and Sr2+ ions. Toxicological & Environmental Chemistry 97 (2015) 11 – 21. |
| [110] | Bikit, I., Mrdja, D., Bikit, K., Grujic, S., Knezevic, D., Forkapic, S. and Kozmidis-Luburic, U.: Radon adsorption by zeolite. Radiation Measurements 72 (2015) 70 – 74. |
| [111] | He, K., Chen, Y., Tang, Z. and Hu, Y.: Removal of heavy metal ions from aqueous solution by zeolite synthesized from fly ash. Environmental Science and Pollution Research 3 (2016) 2778 – 2788. |
| [112] | Kyzioł-Komosińska, J., Rosik-Dulewska, C., Franus, M., Antoszczyszyn-Szpicka, P., Czupioł, J. and Krzyżewska, I.: Sorption Capacities of Natural and Synthetic Zeolites for Cu(II) Ions. Polish Journal of Environmental Studies 24 (2015) 1111 – 1123. |
| [113] | Corma, A.: State of the art and future challenges of zeolites as catalysts. Journal of Catalysis 216 (2003) 298 – 312. |
| [114] | Bandura, L., Franus, M., Józefaciuk, G. and Franus, W.: Synthetic zeolites from fly ash as effective mineral sorbents for land-based petroleum spills cleanup. Fuel 147 (2015) 100 – 107. |
| [115] | Koshy, N. and Singh, D.N.: Fly ash zeolites for water treatment applications. Journal of Environmental Chemical Engineering 4 (2016) 1460 – 1472. |
| [116] | Celik, F.E., Kim, T.J. and Bell, A.T.: Effect of zeolite framework type and Si/Al ratio on dimethoxymethane carbonylation. Journal of Catalysis 270 (2010) 185 – 195. |
| [117] | Jiang, J., Yun, Y., Zou, X., Jorda, J.L. and Corma, A.: ITQ-54: a multi-dimensional extra-large pore zeolite with 20 X 14 X 12-ring channels. Chemical Science 6 (2015) 480 – 485. |
| [118] | Nizami, A.S., Ouda, O.K.M., Rehan, M., El-Maghraby, A.M.O., Gardy, J., Hassanpour, A., Kumar, S. and Ismail, I.M.I.: The potential of Saudi Arabian natural zeolites in energy recovery technologies. Energy xxx (2015) 1 – 10. |
| [119] | Borai, E.H., Harjulab, R., Malinenb, L. and Paajanenb, A.: Efficient removal of cesium from low-level radioactive liquid waste using natural and impregnated zeolite minerals. Journal of Hazardous Materials 172 (2009) 416 – 422. |



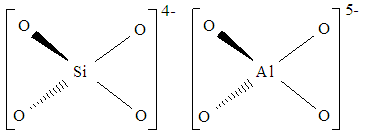
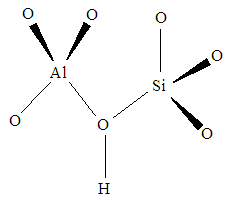
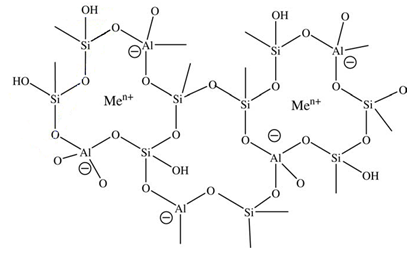
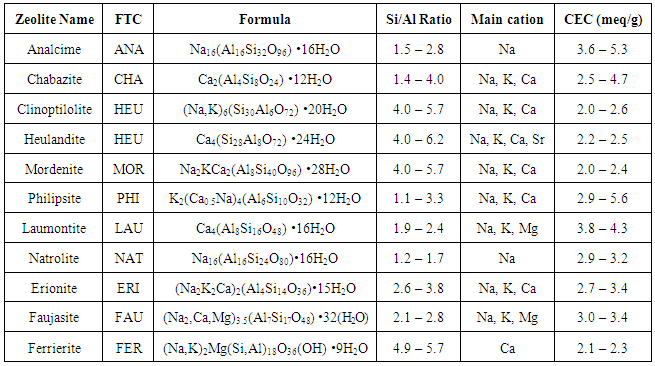
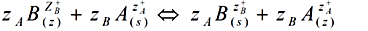 Reaction 3.1Where: zA and zB represent the A & B exchangeable ion charges and the coefficients (z) and (aq) refer to zeolite and to aqueous solution, respectively. The reaction proceeds until equilibrium is attained. The point of equilibrium is defined largely by the theoretical cation exchange capacity (TCEC) of the zeolite, which in turn, is given by the sum of free extra-framework cations of the zeolite. This number of free extra-framework cations of the zeolite is directly related to the amount of aluminum present in the framework and hence the amount of Al3+ that replaces Si4+ in the structure. Cation exchange capacity is usually expressed in milliequivalents (meq) metal per 100g of zeolite. Since the exchangeable cations of natural zeolites are usually represented by: Na+, K+, Mg2+, and Ca2+, for a particular natural zeolite, TCEC = Σ (Na, K, Mg, Ca) as is shown on Table 3.1 [60-63].
Reaction 3.1Where: zA and zB represent the A & B exchangeable ion charges and the coefficients (z) and (aq) refer to zeolite and to aqueous solution, respectively. The reaction proceeds until equilibrium is attained. The point of equilibrium is defined largely by the theoretical cation exchange capacity (TCEC) of the zeolite, which in turn, is given by the sum of free extra-framework cations of the zeolite. This number of free extra-framework cations of the zeolite is directly related to the amount of aluminum present in the framework and hence the amount of Al3+ that replaces Si4+ in the structure. Cation exchange capacity is usually expressed in milliequivalents (meq) metal per 100g of zeolite. Since the exchangeable cations of natural zeolites are usually represented by: Na+, K+, Mg2+, and Ca2+, for a particular natural zeolite, TCEC = Σ (Na, K, Mg, Ca) as is shown on Table 3.1 [60-63].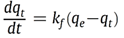
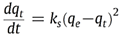
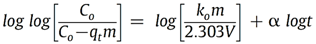

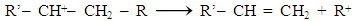
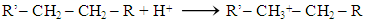
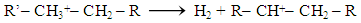

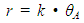
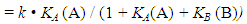
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML