-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2017; 7(5): 185-195
doi:10.5923/j.materials.20170705.11

Covalent Organic Frameworks: Synthesis and Applications
Ayawei Nimibofa1, Ebelegi Augustus Newton1, Ekubo Allen Tobin2, Wankasi Donbebe1
1Department of Chemical Sciences, Niger Delta Universities, Wilberforce Island, Yenagoa, Nigeria
2Department of Chemistry, Federal University, Otuoke, Nigeria
Correspondence to: Ayawei Nimibofa, Department of Chemical Sciences, Niger Delta Universities, Wilberforce Island, Yenagoa, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In comparison with other porous materials, Covalent Organic Frameworks (COFs) have the advantage of low density, large surface area, tunable properties and functionality because of the versatile covalent bonds between organic building units made up of carbon, silicon, oxygen, boron and nitrogen only. The various fields of application leverage on specific characteristic properties of the framework. Gas separation and storage takes advantage of their large surface area and pore volume, they are used in optoelectronics because of the presence of out of plane π-interactions within 2D functionalized COFs, their use as heterogeneous catalysts is because they have inherent network of nanopores, energy storage devices take advantage of their large surface area while their diverse composition and synergistic function makes them invaluable in sensing devices. COFs have also found usefulness in the making of conductive membranes due to their tunable pores and exceptional stability in aqueous media. Inherent large surface area, tunable pores and adjustable functionality makes COFs very alluring candidates for drug delivery systems and separation / enrichment of small molecules.
Keywords: COFs, Topology, Dynamic Covalent Chemistry, Porous organic frameworks, Organic building blocks
Cite this paper: Ayawei Nimibofa, Ebelegi Augustus Newton, Ekubo Allen Tobin, Wankasi Donbebe, Covalent Organic Frameworks: Synthesis and Applications, American Journal of Materials Science, Vol. 7 No. 5, 2017, pp. 185-195. doi: 10.5923/j.materials.20170705.11.
Article Outline
1. Introduction
- Covalent Organic Frameworks (COFs) are porous crystalline polymers put together by linking light elements such as Carbon, Boron, Oxygen and Silicon through strong covalent bonds that are positioned in 2D or 3D topology [1-3]. The usual structure of this unique class of porous material reveals organic building blocks that are topologically connected into broadened lattice arrangements with intermittent skeletons and well ordered cavities [4-6]. In COFs, secondary building units are firmly held by strong covalent bonds between either Carbon –Nitrogen, Boron-Oxygen, Silicon-Carbon or Boron-Oxygen-Silicon, thus the reason for the high stability inherent in them [7, 8]. This special class of Porous Organic Framework (POF) have attracted the interest of researchers on material science because of their versatile functionality, high thermal/chemical stability and large surface area [9, 10].Among the several types of POFs (Aromatic Organic Frameworks, Hypercrosslinked Polymers, Conjugated Microporous Polymers and Covalent Organic Frameworks) COFs have drawn more attention as it is evident in applications such as storage and separation of gases, catalysis, small molecule adsorption/enrichment, optoelectronics, sensing, drug delivery, conductive membranes and energy storage, all because of their intrinsic porosity, well defined aperture tunable pores, large surface area and versatile chemical composition [11-15].COFs also present a combination of exceptional structural and chemical properties such as modularity, crystallinity, porosity, thermal /chemical stability and low density that are hardly found in other porous materials [16-21]. Therefore Covalent Organic Frameworks are becoming excellent candidates for several technological applications owing to their unique structural organic network and controllable structural characteristics [22-24]. This review reports the effectiveness and challenges of various synthetic routes and advances in the application of Covalent Organic Frameworks.
2. Synthesis of Covalent Organic Frameworks
- The invention of COFs in 2005 changed the traditional concept of Porous Organic Frameworks because they were the first successful examples of covalent crystalline porous organic frameworks that possessed well ordered arrangements of mainly organic building units with atomic precision [22]. In the past amorphous forms of organic frameworks were usually synthesized by kinetically controlled coupling reactions that often lead to irreversible formation of covalent bonds with randomly arranged structures that are almost not adjustable. However, this crystallization problem has been surmounted in synthesis and material design through Dynamic Covalent Chemistry (DCC) and Reticular Chemistry respectively [23]. Therefore, nowadays different types of COFs have been synthesized through coherent plan of chemical reactions (Dynamic Covalent Chemistry) and linking of molecular building blocks into extended structures bearing strong covalent bonds [24].Dynamic Covalent Chemistry (DCC)Dynamic Covalent Chemistry is a synthetic strategy utilized by chemists to synthesize Supramolecular assemblies from discrete molecular building blocks [25]. This strategy leads to reversible formation of covalent bonds which can be formed, broken and reformed to finally obtain a stable state [26, 27].
 | Figure 1. Schematic representation of steps in Dynamic Covalent Chemistry [28] |
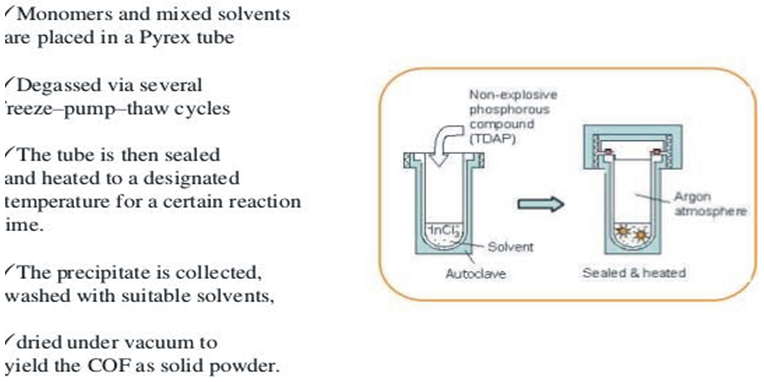 | Figure 2. Schematic diagram showing Solvothermal synthesis of COFs [23] |
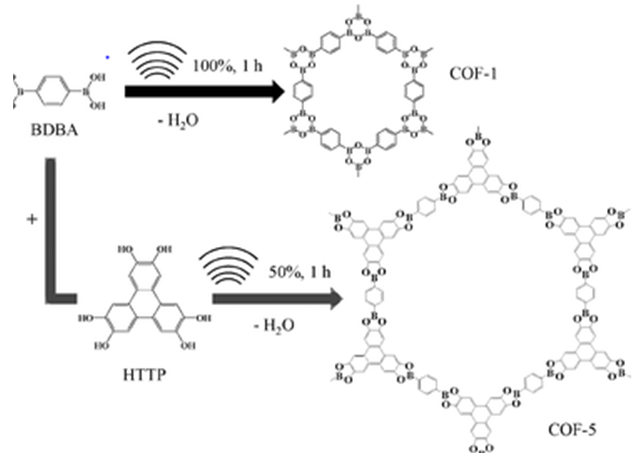 | Figure 3. Diagram showing the synthesis of COF-5 by Sonochemical method [38] |
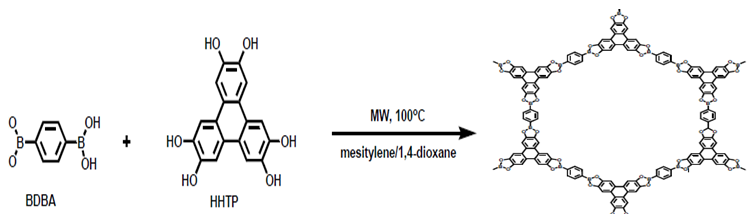 | Figure 4. Schematic diagram showing the synthesis of COF by Microwave synthesis [47] |
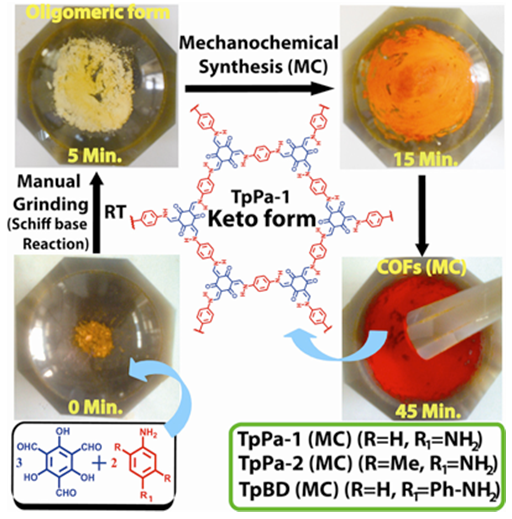 | Figure 5. Diagram showing Mechanochemical synthesis of COFs (TpPa-1 and TpPa-2) [50] |
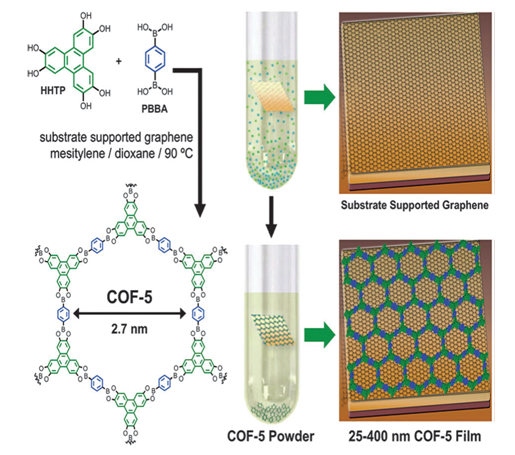 | Figure 6. Schematic presentation of Surface directed synthesis of COFs [56] |
3. Applications of Covalent Organic Frameworks
- The efficient separation and storage of environmentally relevant gases has been a major focus of researchers on porous materials as such almost all the early studies on COFs were focused on separation, storage or sequestration of gases such as CO2, CH4 and H2 [61, 62]. The unique physical and chemical characteristics of COFs makes them outstanding candidates for a plethora of applications such as adsorption, separation and storage of gases [63, 64], catalysis [65, 66], optoelectronics [67, 68], sensors [69] and drug delivery [70].Gas StorageGas adsorption capacity of COFs depends mostly on the constituents and topology of its framework thus, 3D COFs usually outperform their 2D counterparts in this regard because they possess larger surface area and pore volume [71, 72]. Because of their low densities researcher have studied the potential applications of COFs in the adsorption of some gases with respect to clean energy initiatives [73], results of these studies show that COFs are ranked among the most performing porous materials in terms of gas storage capacity [74].H2 StorageFrom transition metal decorated nanotubes, titanium coated single walled nanotubes, Metal Organic Frameworks and now Covalent Organic Frameworks, material scientists have been searching for low cost porous materials with high gravimetric and volumetric density for hydrogen [75-82]. COFs can only function as good hydrogen storage material at very low temperature because they suffer from weak adsorption energies of hydrogen molecules in the framework [83]. Studies have shown that COFs with larger surface area usually have higher hydrogen storage capacity when measured at the same temperature and pressure thus, COF-18 (BET surface area = 1263m2g-1) exhibited the highest hydrogen uptake (1.55 wt% at 1 bar and 77K) when compared to similar 2D COFs [84]. Recent studies have also shown that 3D COFs have superior hydrogen storage capacity when compared to 2D COFs and this has been attributed to their high surface area and low density [85, 86].CH4 StorageMethane is the main component of natural gas that is abundant, inexpensive and a clean source of energy (due to high carbon to oxygen ratio) and ultimately has lower CO2 emission [31, 70]. The effect of adsorbent properties such as surface area, heat of adsorption and heat capacity are very important for effective and safe on-board tanks for storage of natural gas in vehicles [87, 88]. Since COFs are composed of purely organic building blocks, it is possible to surmount technical challenges such as poor adsorption capacity, inefficient charge / heat capacity [89]. Studies have shown that modified forms of COFs have the capacity to achieve the methane storage target of the United States of America’s Directorate of Energy(DOE) (180 cm3 at STP and 35 bars) [90]. Sorption of methane by COFs follows the same pattern as that of hydrogen, 3D COFs possess higher methane sorption capacity than 2D COFs. 3D COF-PCN-14 with BET surface area 1750 m2g-1 has gravimetric methane uptake of 253 mgg-1 at 290 K and 35 bars while 3DCOF-102 with BET surface area of 3620 m2g-1 recorded a gravimetric methane uptake of 187 mgg-1 at 289K and 35 bars and 2D cof-5 with BET surface area of 1670 m2g-1 shows a much lower methane uptake (89 mgg-1) at 298K and 35 bars [91].CO2 StorageAdsorption of CO2 onto porous materials is an energetically efficient and technically feasible technique for CO2 capture and sequestration [89]. The quantity of CO2 uptake by COFs is directly related to the total pore volume thus, COF-102 (pore volume = 1.55cm3g-1) and COF-103 (pore volume = 1.54cm3g-1) exhibit higher CO2 at low pressure than COF-105 (pore volume = 1.07cm3g-1) and COF-108 (pore volume = 0.69cm3g-1) due to compact atomic packing, but at high pressure COF-105 and COF-108 show CO2 storage capacities because of larger pore volumes [92].Although COFs constructed via the formation of Carbon-Nitrogen bonds are said to have enhanced chemical robustness [93], they typically possess moderate specific surface areas and Mesoporous pores which restricts their use as effective gas adsorbents [95-97]. The synthesis of new COFs with high crystallinity, robust chemical stability, excellent porosity, high surface area and small pore size for CO2 storage has been reported thus, the synthesis of a new azine-linked Covalent Organic Framework (ACOF-1) with high surface area and large pore size with CO2 storage capacity of 177mgg-1 at 273 and 1bar [98]. Similarly, lithium doping has been reported to be the most effective means of enhancing the CO2 storage capacity of COFs thus, lithium doped COF-102 showed CO2 storage capacity of 409mgg-1 [99, 100].NH3 StorageStudies have shown that molecularly designed COFs bearing functional groups on their walls can interact with gas molecules [101]. COFs with boron-ester linkages contain high density Lewis acid boron sites that can strongly interact with Lewis bases and this makes them ideal adsorbents and storage materials for corrosive chemicals like ammonia [102]. COF-10 exhibited the highest ammonia uptake (15 molKg-1) at 298K and 1 bar when compared to other porous materials such as 13X Zeolite, amberlite and Mesoporous silica [103]. Reports also reveal that adsorbed ammonia can be removed from the pores of COF-10 via heating at 200°C under vacuum and the COF can be recycled several times without much deterioration in its adsorptive capacity [104, 105]. The exceptional high uptake of ammonia by COF-10 has been attributed to the formation of a classical ammonia-borane coordination bond.OptoelectronicsCOFs show unique optical and electrical properties when functionalized with photoelectric moieties [106]. Out of plane π – interactions in 2D COFs serve as primary driving forces for the formation of layered structures that induce huge electronic coupling between π – orbitals of stacking layers, this in turn facilitates the transportation of charge carriers and excitons (photo excited states) through organized and inbuilt pathways [107]. The application of pyrene-functionalized COFs in semi conduction and photoconduction has been exemplified by Tp-COF in its P-type semi conductive character with photon harvesting capacity in the UV-Visible regions and Ppy-COF in its photoconductivity and quick response to light irradiation [108].Heterogeneous CatalysisPorous organic polymers have emerged as new candidates for efficient heterogeneous catalysis due to their ability to load various molecules onto their nanopores [99, 109]. Imine-based COF-LZU1 loads Palladium ions into its pores through a coordination reaction between atoms of nitrogen in the COF and Palladium ions to form Pd / COF-LZU1.Pd / COF-LZU1 produced excellent catalyst performance when tested in the catalysis of Suzuki-Miyaura coupling reaction [68, 110]. In like manner, COF-102 entraps ferrocene molecules in huge amounts (about 42% of its total pore volume) through sublimation of ferrocene into COF-102 channels under vacuum [7]. It has been reported that Cobalt Porphyrin COF (COF-366 Co) catalyzed the electrochemical reduction of CO2 with promising activity and selectivity to a competing reaction (H2 formation) [111].Energy StorageBecause of their high surface area COFs have the special ability of incorporating redox-active groups which makes them good candidates as electrochemical capacitors [112, 113]. Studies have shown that electro-polymerizing 3,4- ethylendioxythiophene (EDOT) within the pores of DAAQ-TFP COF films showed exceptional electrochemical feat as the conductive additive increased the volumetric capacitance by accessing inactive quinones and enabling charge cycling at high C-rates with minimal loss of capacitance [114].Conductive Membranesβ -ketoenamine-linked COFs have been identified as potential candidates for proton-conductive membranes in fuel cells because of their tunable pores and exceptional stability in aqueous acid [115]. Studies have also shown that an azobenzene containing β-ketoenamine-linked COF doped with phosphoric acid exhibited remarkable proton conductivity near room temperature. In like manner a triazol-functionalized Imine-linked COF had conductivity of 1.1X10-4 Scm-1 at 130°C [116].Drug DeliveryAlthough the application of COFs for drug delivery is still at its early stage great effort have been made to develop COF-based drug delivery systems, two 3D poly Imide COFs PI-COF-4 and PI-COF-5 were the very first Covalent Organic Frameworks applied for drug delivery as they showed very good loading and well controlled release profiles for Iboprofen [117]. Further studies on the potentials of COFs in drug delivery revealed how COF surfaces could be destructed under ultra violet irradiation through isomerization and the decomposed COF surface could be recovered after allowing it to anneal, because the condensation of boronic acid is reversible thus, this reversible photo-induced decomposition recovery of the COF shows controlled loading and release of copper phthalocyanine [118].SensingRecent research reports have demonstrated sensing applications of Covalent Organic Frameworks and this can be attributed to their diverse compositions and synergistic functionality. The very first report on the use of COFs in chemo- sensing revealed that an azine -linked COF possessed notable sensitivity and selectivity in the detection of 2,4,6-trinitrophenol explosive [68]. Similarly, a thioether-based fluorescent Covalent Organic Framework (COF-LZU8) has been applied for the removal of Hg+2 from water [119].Separation / Enrichment of Small MoleculesCOFs have attracted so much attention in the adsorption of small molecules because of their tunable pores, large surface area and adjustable functionality. A benzimidizole-functionalized 2D COF containing carboxylic groups has been used as a matrix for solid state extraction in the separation and enrichment of uranium [120]. Furthermore, a hydrazone COF-based extraction system has been developed for enrichment and analysis of trace sudan dyes in Chilli powder and sausage samples with low detection limits [120].
4. Conclusions
- Covalent Organic Frameworks (COFs) are an emerging unique class of porous crystalline materials. They are synthesized purely from organic building units that are linked by irreversible covalent bonds creating highly ordered and pre-designable two dimension and three dimension architecture. One major hinderance to the practical applicabitity of COFs is their relatively poor moisture tolorance that is ascribed to the reversibilty of covalent linkages formed by borate and boroxine linkers which are prone to cleavage when exposed to moisture [121].Therefore, the development of new synthetic methods, exploration of moisture tolorant linkers and new linkage reactions have facilitated the expansión of the COF family. The detection of structural defects in COFs and improved understanding of the thermodynamic behavior of Dynamic Covalent Chemistry during condensation reactions would enable the consistent preparation of high quality COF materials. In comparison with other porous materials like zeolite and hybrids of MOFs, COFs have the advantage of low density, large surface area, tunable properties and functionality because of the versatile covalent bonds between organic building units made up of carbon, silicon, oxygen, boron and nitrogen only. This review has highlighted several synthetic strategies and applications of COFs, with each application leveraging on specific characteristic property of the framework. The various fields that have enjoyed the patronage of COFs include; gas separation and storage which takes advantage of their large surface area and pore volume, they are used in optoelectronics because of the presence of out of plane π-interactions within 2D functionalized COFs, the utilization of COFs in heterogeneous catalysis is borne out of the fact that they have inherent network of nanopores, energy storage devices take advantage of the large surface area of COFs while they are used in sensing devices because of their diverse composition and synergistic functions. COFs have also found usefulness in the making of conductive membranes due to their tunable pores and exceptional stability in aqueous media. Inherent large surface area, tunable pores and adjustable functionality makes COFs very alluring candidates for drug delivery systems and separation / enrichment of small molecules. Although advances have been recorded in the synthesis and applications of Covalent Organic Frameworks the challenge of synthesizing functional COFs with highly periodic, stable and robust framework needs to be surmounted.
Future Perspective
- Considering the numerous applications of porous materials such as Layered Doubled Hydroxides (LDH), Metal Organic Frameworks (MOFs), Liposomes and dendrimers, COFS could also be used for corrosion inhibitors [122], adsorption of heavy metals [123] and formation of nanocomposites [124].
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML