-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2017; 7(4): 71-77
doi:10.5923/j.materials.20170704.01

EPR Characterization of Ferroelectric Ceramics (Sb, Cu)-Doped BaTiO3
Said Daoudi1, Lahcen Bejjit1, Mustapha Haddad1, El Mostapha Yahiaoui1, Lahcen Bih2, Faouzi Bensamka3, Ahmed Outzourhit3
1Laboratoire de Spectrométrie des Matériaux et Archéomatériaux (LASMAR - URAC11), Faculté des Sciences, Université Moulay Ismail, B.P. Zitoune, Meknès, Morocco
2Equipe physico-chimie de la matière condensée, Faculté des Sciences, Université Moulay Ismail, B.P. Zitoune, Meknès, Morocco
3Laboratoire des Sciences des Matériaux, Faculté des Sciences Semlalia, BP, Université Cadi Ayyad, Marrakech, Morocco
Correspondence to: Said Daoudi, Laboratoire de Spectrométrie des Matériaux et Archéomatériaux (LASMAR - URAC11), Faculté des Sciences, Université Moulay Ismail, B.P. Zitoune, Meknès, Morocco.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This work presents the results of an EPR study of BaTiO3 (BTO) ferroelectric ceramics pure and doped with antimony and copper. This study revealed the nature of the defects created in pure BTO and those generated by the effect of dopant. The computer simulation of the EPR spectra showed that these spectra are composed of several signals related with the presence of the paramagnetic centers in the doped and undoped BTO structure. These centers are identified, by determining their EPR parameters, as defects 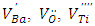 and Ba.
and Ba.
Keywords: Ceramics, Electron paramagnetic resonance (EPR), Structural defects
Cite this paper: Said Daoudi, Lahcen Bejjit, Mustapha Haddad, El Mostapha Yahiaoui, Lahcen Bih, Faouzi Bensamka, Ahmed Outzourhit, EPR Characterization of Ferroelectric Ceramics (Sb, Cu)-Doped BaTiO3, American Journal of Materials Science, Vol. 7 No. 4, 2017, pp. 71-77. doi: 10.5923/j.materials.20170704.01.
Article Outline
1. Introduction
- Perovskite-type BaTiO3 ceramics have many applications in various domains, especially in electronics and telecommunications because of their piezoelectric, pyroelectric and ferroelectric properties [1-7]. To improve the properties of these materials and adapt them to specific applications, several doping elements have been added to BaTiO3 structure [8-17]. The obtained ferroelectric ceramics have been characterized by different method [18-20]. In last years, many works have focused on the defects created by doped BTO using electron paramagnetic resonance (EPR), a powerful tool for identifying paramagnetic elements and crystalline defects. Kolodiazhnyi and Petric [21], Dunbar et al. [22] and Castro et al. [23] have associated EPR singlet, with g values between 1.973 and 1.974, to the singly ionized barium vacancy
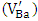 , and room temperature investigations [21] revealed the same signal in both of doped and undoped BaTiO3, after oxidation of reduced ceramics in air. The g = 2.004 EPR signal has been attributed to the titanium vacancy with unpaired electron spin, i.e.
, and room temperature investigations [21] revealed the same signal in both of doped and undoped BaTiO3, after oxidation of reduced ceramics in air. The g = 2.004 EPR signal has been attributed to the titanium vacancy with unpaired electron spin, i.e. 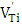 or
or 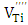 . Recently, Lu et al. [16, 17] have identified several paramagnetic point defects in Pr-doped BaTiO3 and La, Tb co-doped BaTiO3 ceramics. In the first material the authors have associated EPR singlet, with g = 2.002 to the singly ionized Ti-vacancy defects, the signal at g= 1.932 to Ti3+ (3d1) and the signal at g= 1.974 to Ba vacancies. In the second material the EPR signals at g = 2.004 and g=1.974 have been attributed to V(Ti) and V(Ba) defects respectively.In this paper, we have studied the effect of antimony and copper dopants on the BaTiO3 (BTO) composition. Using EPR technique, we tried to identify ionized barium, titanium and oxygen vacancies produced in doped BaTiO3 lattice by Sb and Cu elements.
. Recently, Lu et al. [16, 17] have identified several paramagnetic point defects in Pr-doped BaTiO3 and La, Tb co-doped BaTiO3 ceramics. In the first material the authors have associated EPR singlet, with g = 2.002 to the singly ionized Ti-vacancy defects, the signal at g= 1.932 to Ti3+ (3d1) and the signal at g= 1.974 to Ba vacancies. In the second material the EPR signals at g = 2.004 and g=1.974 have been attributed to V(Ti) and V(Ba) defects respectively.In this paper, we have studied the effect of antimony and copper dopants on the BaTiO3 (BTO) composition. Using EPR technique, we tried to identify ionized barium, titanium and oxygen vacancies produced in doped BaTiO3 lattice by Sb and Cu elements. 2. Experimental Procedures
2.1. Samples Preparation
- Perovskite ceramics of BaTiO3 type were prepared by the hydrothermal method [24]; Barium hydroxide Ba(OH)2 is prepared by dissolving barium oxide BaO in distilled water. A mixture of Ba(OH)2, TiO2, Sb5+ or Cu2+ aqueous solution was introduced in an autoclave. The media is rendered alkaline by adding sodium hydroxide NaOH. Then, it is heated to 140°C and maintained at this temperature for 14 hours. The obtained product is filtered, washed to eliminate Na+ ions and dried at 100°C.
2.2. EPR and XRD Measurements
- The EPR spectra X-band (9.5 GHz) were recorded at room temperature on powder samples, using a Bruker spectrometer. The numerical simulation of the experimental spectra was carried out by developing a computational program well adapted to the nature of the paramagnetic centers studied in this work. The X-ray diffraction (XRD) studies were performed in a set-up equipped with a Siemens M386-X-A3 goniometer using the CuKα radiation.
3. Results and Discussions
3.1. Structural Properties
- Barium titanate (BaTiO3) ceramics were analyzed by X-ray diffraction (XRD) [24]. Figure 1 shows typical XRD diagrams of the dried BaTiO3 powders prepared at two different reactor temperatures at 120 and 140°C. Theses diagrams show the presence of the characteristic peaks of the cubic phase of BaTiO3. A secondary phase identified as anatase TiO2 is also observed. One can notice that the amount of these impurity phase decreases as the reactor temperature is increased from 120 to 140°C.
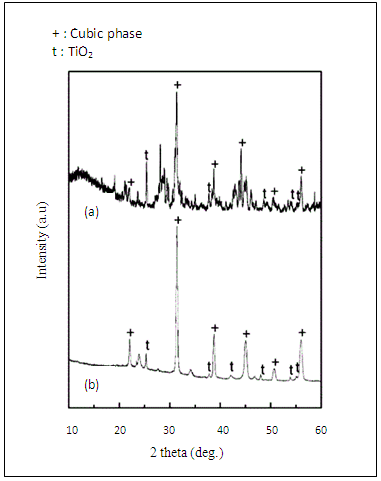 | Figure 1. XRD patterns of untreated BaTiO3 powders prepared at: (a) 120°C; and (b) 140°C |
3.2. EPR Results
- For each sample we give the experimental and the calculated spectrum. The numerical simulation of the experimental spectra was carried out using a spin Hamiltonian composed of electronic Zeeman and hyperfine terms: Ĥ =βSgH + SAIH is the magnetic field strength, A is the hyperfine constant, S and I are electronic and magnetic spin respectively. g is the gyromagnetic factor calculated by the relationship hν0=gβH, where h is the plank constant (6,626 × 10-34 J s), ν0 is the microwave frequency and β is the Bohr magnetron (9.262× 10 −24 J/T).
3.2.1. BaTiO3 Undoped
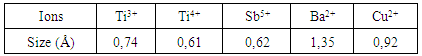
- The EPR spectrum from the undoped BaTiO3 sample is shown in figure 2. The calculated spectrum allowed us to identify several types of paramagnetic centers (Table 1). The hyperfine structure with six lines is due to Mn2+ (3d5, 6S5/2) and the signal with g = 2,000 (relatively high intensity) is attributed to the Fe3+ (3d5, 6S5/2), both of these elements are the main impurities associated with titanium in the BaTiO3 [11, 16, 21, 23]. The low intensity signals at g = 2.004 and g =1.974 are attributed respectively to titanium vacancies
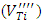 [9] and barium vacancies
[9] and barium vacancies 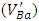 [16, 21, 23]. The low intensities of these signals can be associated with small quantities of
[16, 21, 23]. The low intensities of these signals can be associated with small quantities of 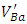 and
and 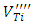 in the undoped BaTiO3 ceramics [21, 25].
in the undoped BaTiO3 ceramics [21, 25].
|
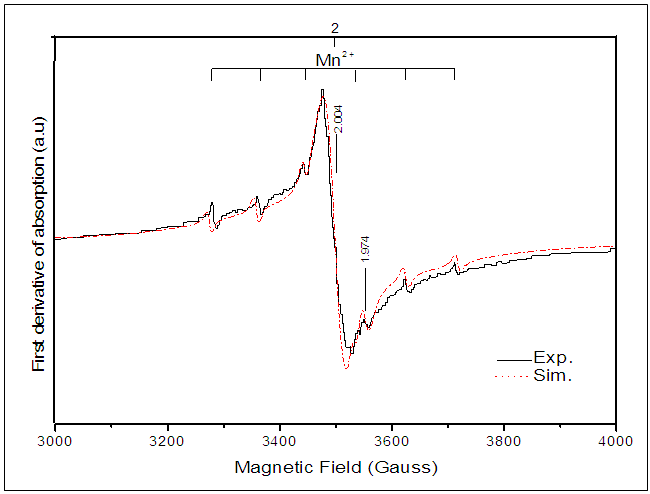 | Figure 2. Experimental and calculated EPR spectrum of BaTiO3 |
3.2.2. EPR Spectrum of Sb-doped BaTiO3
- Figure 3 shows an experimental and calculated EPR spectrum of BaTiO3 doped with 0.3% antimony. In addition to signals previously detected in the non-doped BaTiO3 spectrum (Table 1), we observe a hyperfine structure with 4 lines around g//=2.38 and g┴=2.063 [26]. This hyperfine structure corresponds probably to the Ba+ center (Ba2+ + 1e- →Ba+) which is a reduced ion by the electrons coming from oxygen vacancies according to the following equation [26, 27]:
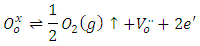 As it is well known, barium has two isotopes, 135Ba and 137Ba, which have the same nuclear spin (I = 3/2), and with natural abundances of 6.59% and 11.32%, respectively. This is what justifies that the EPR spectra show a hyperfine structure with 4 lines which are not clearly distinguished. On the other hand, comparing the spectra from undoped BaTiO3 and Sb-doped BaTiO3, the signal related to the barium vacancies increases significantly. This could be give information about the site occupied by antimony. In fact, the increasing intensity of this signal indicates that the sites associated to barium vacancies
As it is well known, barium has two isotopes, 135Ba and 137Ba, which have the same nuclear spin (I = 3/2), and with natural abundances of 6.59% and 11.32%, respectively. This is what justifies that the EPR spectra show a hyperfine structure with 4 lines which are not clearly distinguished. On the other hand, comparing the spectra from undoped BaTiO3 and Sb-doped BaTiO3, the signal related to the barium vacancies increases significantly. This could be give information about the site occupied by antimony. In fact, the increasing intensity of this signal indicates that the sites associated to barium vacancies 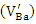 are created with Sb-doping of BTO. This could be explained by the fact that the antimony ion introduces the BTO network by occupying the Ti4+ site. Indeed, according to the ionic radius of the elements existing in the structure (Table 2), we see that the antimony Sb5 + can occupy the Ti4 + site. The mechanism of the formation of these barium vacancies is represented by the following equations [23]:
are created with Sb-doping of BTO. This could be explained by the fact that the antimony ion introduces the BTO network by occupying the Ti4+ site. Indeed, according to the ionic radius of the elements existing in the structure (Table 2), we see that the antimony Sb5 + can occupy the Ti4 + site. The mechanism of the formation of these barium vacancies is represented by the following equations [23]: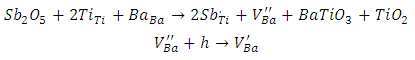
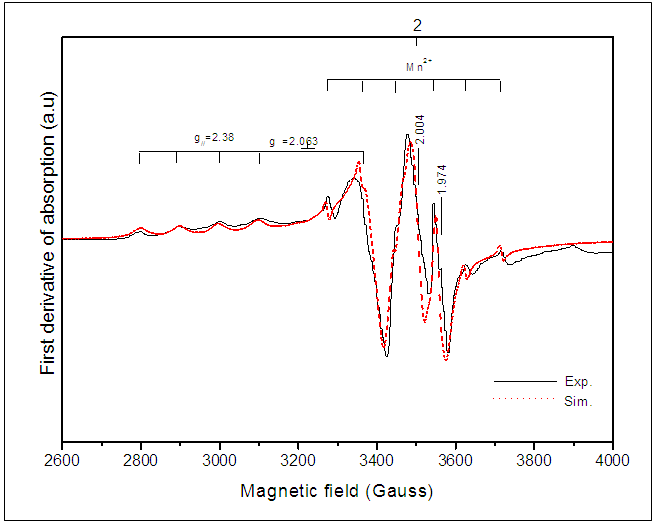 | Figure 3. Experimental and calculated EPR spectrum of 0.3% Sb-doped BaTiO3 |
|
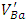 species produced by the double ionization of
species produced by the double ionization of 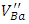 are paramagnetic [23].In order to study the effect of doping content on the shape of the EPR spectrum and the created vacancies, the EPR spectra of the undoped, 0.3% Sb-doped and 1% Sb-doped BaTiO3 are presented together (Figure 4). By normalizing and comparing the spectra of 0.3% Sb and 1% Sb, we can observe two opposite effects when the concentration of the Sb increases: (i) the intensity of signals associated to
are paramagnetic [23].In order to study the effect of doping content on the shape of the EPR spectrum and the created vacancies, the EPR spectra of the undoped, 0.3% Sb-doped and 1% Sb-doped BaTiO3 are presented together (Figure 4). By normalizing and comparing the spectra of 0.3% Sb and 1% Sb, we can observe two opposite effects when the concentration of the Sb increases: (i) the intensity of signals associated to 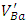 and Ba+ centers decreases, (ii) the intensity of
and Ba+ centers decreases, (ii) the intensity of 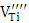 center increases. This means that the doping content has a significant effect on the nature of the created paramagnetic centers in the BaTiO3 structure. These results are in good agreement with the mechanism proposed by Castro et al. [23], to describe the creating defects in Sb-doped BaTiO3:-For the low rate 0.3% Sb:
center increases. This means that the doping content has a significant effect on the nature of the created paramagnetic centers in the BaTiO3 structure. These results are in good agreement with the mechanism proposed by Castro et al. [23], to describe the creating defects in Sb-doped BaTiO3:-For the low rate 0.3% Sb: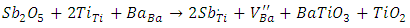 -For high rate 1% Sb:
-For high rate 1% Sb: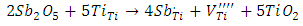
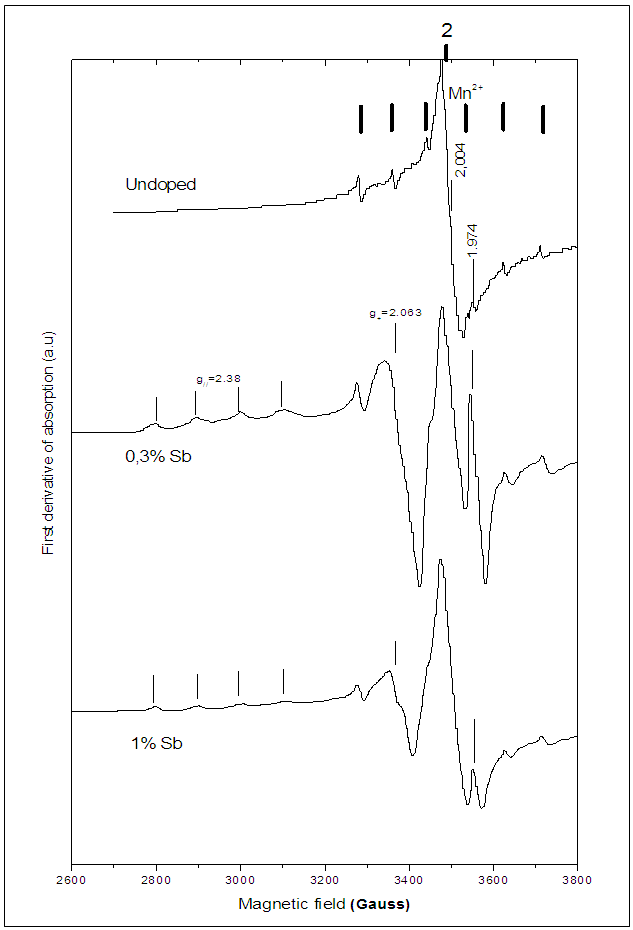 | Figure 4. EPR Spectra of undoped and Sb-doped BaTiO3 |
3.2.3. EPR Spectra Cu-doped BaTiO3
- EPR spectra are recorded for 0.3% and 1% Cu-doped BaTiO3. All the identified EPR signals in undoped BTO are present in doped samples. However, the Cu-doping induces the appearance of new signals. The calculated spectrum for 1% Cu-doped (Figure 5) allowed us to distinguish several lines, three hyperfine structures around (g//=2.35 and g┴=2.096), (g//=2.3 et g┴=2.027) and (g//=2.38 and g┴=2.063); the two first hyperfine structures are attributed to Cu2+ ions in two different sites [28] and the third one is attributed to Ba+ ions. The weak line at g = 1.963 is attributed to oxygen vacancies [23].
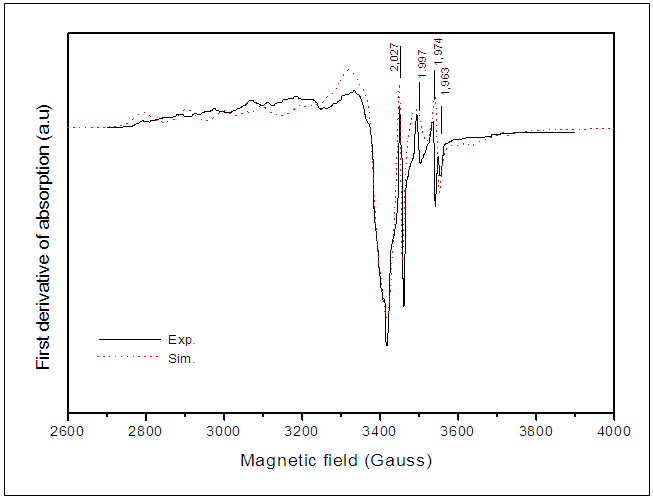 | Figure 5. Experimental and calculated EPR spectrum of 1% Cu-doped BaTiO3 |
 established by Langhammer et al. [28], the copper can occupy titanium site. In the other hand, the comparison of EPR spectra from 0.3% and 1% Cu-doped BaTiO3 samples (Figure 6) revealed that as copper content increases, Cu2+ signals increase and the intensity of the signal (g = 1.963) associated with
established by Langhammer et al. [28], the copper can occupy titanium site. In the other hand, the comparison of EPR spectra from 0.3% and 1% Cu-doped BaTiO3 samples (Figure 6) revealed that as copper content increases, Cu2+ signals increase and the intensity of the signal (g = 1.963) associated with  increases.
increases. 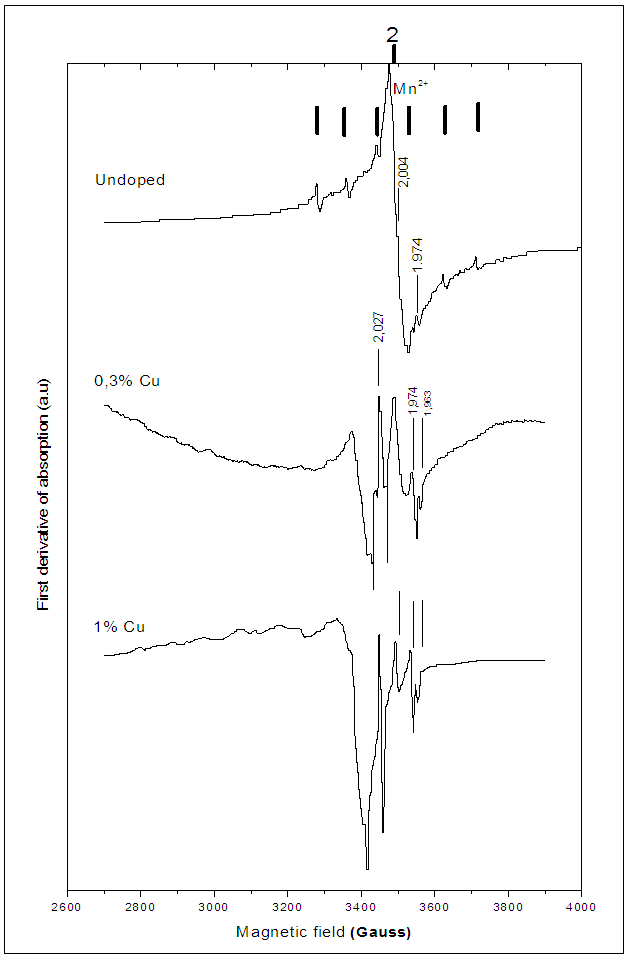 | Figure 6. EPR spectra of undoped and Cu-doped BaTiO3 |
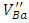 defects which can be described by the equation
defects which can be described by the equation 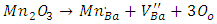 and the weakness of Mn2+ signal intensity could be explained by the process of oxidation-reduction of manganese present in BaTiO3 as impurity, according to the following reaction:
and the weakness of Mn2+ signal intensity could be explained by the process of oxidation-reduction of manganese present in BaTiO3 as impurity, according to the following reaction: 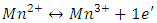
4. Conclusions
- The Sb-BTO and Cu-BTO ceramics are prepared by the hydrothermal method and are studied by EPR. Their experimental and simulated spectra showed the presence of several paramagnetic centers, namely
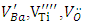 and Ba+. The defects identified depend on the nature of the dopant; those identified in Sb-BTO are
and Ba+. The defects identified depend on the nature of the dopant; those identified in Sb-BTO are 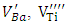 and Ba+ and those contained in Cu-BTO are
and Ba+ and those contained in Cu-BTO are 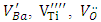 , Ba+ and Cu2+. It’s also found in Sb-BaTiO3 ceramics that the intensity of
, Ba+ and Cu2+. It’s also found in Sb-BaTiO3 ceramics that the intensity of 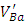 and Ba+ centers decreases and the intensity of
and Ba+ centers decreases and the intensity of 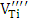 center increases with Sb doping of BaTiO3.
center increases with Sb doping of BaTiO3. Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML