-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2017; 7(3): 64-70
doi:10.5923/j.materials.20170703.04

Characterization of Pectin Biofilms with the Addition of Babassu Mesocarp and Whey Protein Concentrate
Lopes I. A.1, Santos Jr J.1, Da Silva D. C.1, Da Silva L. J. S.1, Barros A. K.2, Villa-Vélez H. A.1, Santana A. A.1
1Chemical Engineering Coordination, Federal University of Maranhão, São Luís, Brazil
2Department of Electrical Engineering, Federal University of Maranhão, São Luís, Brazil
Correspondence to: Villa-Vélez H. A., Chemical Engineering Coordination, Federal University of Maranhão, São Luís, Brazil.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Biofilms are defined as flexible films prepared from biological materials with the potential for application in the pharmaceutical and food areas, their use depending on various parameters such as the mechanical properties, barrier properties and water solubility, amongst others. The present work aims to characterize pectin (P) biofilms composed of babassu mesocarp (BM) and whey protein concentrate (WPC), prepared by the casting method. Thus physical attributes such as moisture content, water soluble mass, thickness, water vapor permeability and degradation were determined. Fourier-transform infrared spectroscopy and scanning electron microscopy were used in order to determine the chemical composition and surface structure, respectively, of the biofilms. Calcium crosslinked and non-reticulated films were considered. The biofilms composed of 100%P and 50%P+50%WPC were more hygroscopic than those composed of 50%P+50%BM and 50%P+25%BM+25%WPC. The lowest water vapor permeability and solubility values were found for the films plasticized with 50%P+50%BM. The films plasticized with 50%P+50%BM and 50%P+25%BM+25%WPC showed very similar functional attributes regarding their application as food wrappings and/or production bags.
Keywords: Babassu mesocarp, Casting method, Physical characterization, Whey protein concentrate, Glycerol
Cite this paper: Lopes I. A., Santos Jr J., Da Silva D. C., Da Silva L. J. S., Barros A. K., Villa-Vélez H. A., Santana A. A., Characterization of Pectin Biofilms with the Addition of Babassu Mesocarp and Whey Protein Concentrate, American Journal of Materials Science, Vol. 7 No. 3, 2017, pp. 64-70. doi: 10.5923/j.materials.20170703.04.
Article Outline
1. Introduction
- Biofilms are natural polymers formed from biological, animal or vegetable materials such as microorganisms, lipids, proteins and polysaccharides. These materials, obtained from the growth of microorganisms or from animal protein and plants, are currently replacing the plastic materials synthetized from petroleum due to their environmental friendliness [1, 2]. Recently, the production of biofilms combining different polysaccharides, proteins and lipids has been the focuse of research to improve their functionality (e.g., mechanical and barrier properties), showing synergistic advantages when compared with the pure components [3].Pectin is an anionic polysaccharide widely used in the food industry due its high availability in citrus peels, with low extraction costs, high solubility, good gelling properties, high biocompatibility and simple modification by chemical and biochemical processes. In addition, pectin has great potential for use in the preparation of biofilms, as food coverings and drug coating, amongst others [4, 5]. The characteristics of biofilms composed of pectin have been reported by Giancone et al., [6], where films made from high methoxyl pectin showed barrier properties comparable to commercial biodegradable film packaging and, according to Fishman et al., [7], where films made from mixtures of pectin/starch/plasticizer resulted in a very definite loss of brittleness of the film, making them much more flexible. Thus the physical, thermal and mechanical properties of pectin-based films, depends on the aggregated substances, such as plasticizers and emulsifiers, as well as the coverings used on the matrix.Amongst the diversity of raw material that have been researched to produce biofilms, the babassu mesocarp and whey protein concentrate are two possible compounds that can be mixed with the filmogenic matrix containing pectin. The babassu (Orbignya phalerata Mart.) mesocarp is produced during the separation of the babassu kernel, being widely marketed in the states of Maranhao, Piaui and Tocantins (Brazil) [8]. The dried babassu mesocarp flour is used as a substitute for cassava flour, and serves as food for both humans and animals [9], as well as in natural medicines for the treatment of multiple diseases [10-12]. Chemically, the babassu mesocarp flour contains 60% of starch (gelatinization temperature of the granules in the range from 63 to 73°C) [13] and a significant amylose content and its polymeric structure is highly crystalline, showing promising applications in the form of elastic matrices [14, 15]. On the other hand, the whey protein concentrate contains globular proteins such as β-lactoglobulin, α-lactoalbumin and bovine serum albumin, presenting both thermo-reversible and thermo-irreversible gels when heated above 65-70°C, with wide applications for use in the food industry as an emulsifier [16]. Moreover, the emulsifying capacity depends on the composition of the matrix considered (e.g., ionic strength and pH), the processing to which it is submitted and the storage conditions that it experiences during its lifetime, e.g., heating, cooling, mechanical agitation [17, 18].Both compounds show promising applications in the production of biofilms, since films prepared with pure pectin are completely soluble in water, besides having poor mechanical properties. Thus the combinations could provide new characteristics which should be researched. In addition, studies concerning the use of babassu and whey protein concentrate in the confection of biofilms have not been reported in the literature. Thus the present work aims to study the influence of the mixture of pectin/babassu mesocarp/whey protein concentrate, with added calcium chloride (crosslinking agent) and glycerol (plasticizing agent), on the chemical, barrier and mechanical properties of biofilms.
2. Material and Methods
2.1. Raw Materials and Sample Preparation
- Citric pectin (P) (Isofar, Rio de Janeiro, Brazil), whey protein concentrate (WPC) (Alibra, Campinas, Brazil), anhydrous calcium chloride (Merck, Darmstadt, Germany), glycerol (Synth, São Paulo, Brazil) and babassu mesocarp (BM) were used in this research.Babassu mesocarp (BM) was obtained from in nature coconut. The coconut was first peeled and the fiber and mesocarp extracted with a steel knife. The mesocarp was then milled in a ball mill (Model 460*600, Yongsheng, China) and sieved through a No. 400 mesh screen (Tyler series, W.S. Tyler, USA) in order to obtain a powder with a diameter of 37 μm. The chemical characteristics of the BM were determined according to the Standard Analytical Methods [19], obtaining values for moisture content of 1.66±0.03 g/100g (w.b.), protein content of 4.03±0.58 g/100g (w.b.), fat content of 0.43±0.10 g/100g (w.b.), and ash content of 2.34±0.08 g/100g (w.b.). The carbohydrate content was obtained by difference, obtaining a value of 91.54±0.08 g/100g (w.b.).
2.2. Film Preparation
- Biofilms were prepared according to the methodology of Santana and Kieckbusch [20] with modifications. Citric pectin (P) together with babassu mesocarp (BM) and/or whey protein concentrate (WPC) and glycerol (3 ml) were dissolved in distilled water to a total volume of 200 ml, with constant magnetic stirring. After dissolution, 30 ml of the cross-linking solution was added and the temperature increased to 70°C. The cross-linking solution (Ca2+) was added slowly to the solution containing the citric pectin with babassu mesocarp and/or whey protein concentrate, using a peristaltic pump (model 77120-70, Masterflex, USA) with a maximum flow rate of 0.6 ml/min, to avoid local gelling. The films were obtained by the casting technique, i.e., aliquots of 50 g of the hot solution were poured into round Plexiglas pans (area = 172.03 cm2) and slowly dried at 40°C in an oven with air recirculation (model 099EV, Fanem, Brazil) for 18 to 20 hours. The dried films were removed from the support and stored at 52% RH and 25°C for 48 hours. In all, four formulations were studied, 100%P, 50%P+50WPC, 50%P+50%BM and 50%P+25%BM+25%WPC.
2.3. Characterization of the Biofilms
- Film thickness The thickness of the biofilms (δ) was controlled by pouring a constant mass (50 g) into the film forming solution over the support. The thickness of the conditioned films was measured using a digital micrometer (model MDC-25S, Mitutoyo, Japan). Measurements were taken at five different positions on the film surface and the mean value reported.Moisture contentThe moisture contents (w) of the four formulations were determined in a vacuum oven (model MA030, Marconi, Brazil) at 60°C for 24 h using the gravimetric method AOAC 934.06 [19]. The analyses were carried out in triplicate and reported on a dry weight basis (d.b.).Matter dissolved in the waterThe mass dissolved in the water (S) was determined in triplicate according to the methodology proposed by Irissin-Mangata et al. [21]. The mass of a piece of film was determined and then immersed in 50 ml of distilled water under mild shaking action (175 rpm) at 25 ± 5°C for 24 h, in an orbital controlled temperature shaker (model 3545-40-EA, Termo Fisher Sci Inc, USA). The sample was then transferred to a vacuum oven (60°C for 24 h) to determine the final dry matter. The soluble matter was expressed as a function of the initial dry matter according to Eq. (1):
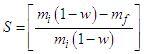 | (1) |
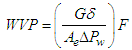 | (2) |
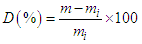 | (3) |
 | Figure 1. Biodegradation analysis: (a) Biofilm packaging composed of 100%P (F1), 50%P+50%WPC (F2), 50%P+50%BM (F3) and 50%P+25%BM+25%WPC (F4) and, (b) biofilms in the natural environment |
2.4. Morphology
- Scanning electron microscopy (SEM) was employed to study the surface and cross-sectional morphologies of the biofilms produced. All the biofilms were dried in a vacuum oven at 70°C for 24 h and the samples mounted onto stubs, sputter coated with gold in a vacuum chamber and photographed (magnifications of x145, 265, 360, 620, 1,450, 3,100, 4,100 and 5,000) using the scanning electron microscope (model Phenom Pro, Phenom-World, Netherland) operated at 5 kV.
2.5. Fourier-transform Infrared Spectroscopy (FTIR)
- FTIR-ATR spectroscopy of the biofilms was carried out using a spectrometer (model IR-Prestige, Shimadzu, Japan) with 128 scans between 400 and 5000 cm-1 and 4 cm-1 resolution.
2.6. Statistical Analysis
- All regressions were carried out using the Statistica V9 (Statsoft, Tulsa, USA) software. In addition, an analysis of variance and Tukey test were employed to determine any statistically significant differences (p < 0.05) between the averages.
3. Results and Discussion
3.1. Biofilm Characterizations
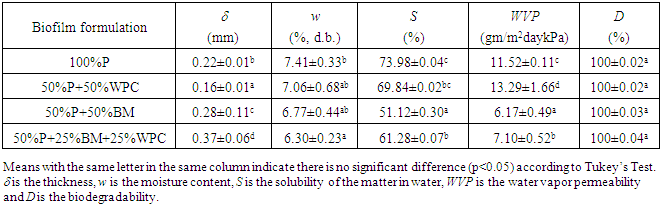
- Biofilms composed of pectin with the addition of babassu mesocarp and/or whey protein concentrate were formulated and prepared by the casting technique, obtaining the following four matrices 100%P, 50%P+50WPC, 50%P+50%BM and 50%P+25%BM+25%WPC. Visually, the 100%P and 50%P+50%WPC biofilms showed a slightly yellowish, translucent and homogeneous surface, flexible texture and were easily removed from the Plexiglas mold. On the other hand, the 50%P+50%BM and 50%P+25%BM+25%WPC biofilms showed a brown homogeneous surface and low flexibility as compared to the biofilms without BM (Fig. 2). Table 1 shows the filmogenic characteristics of thickness, moisture content, the matter dissolved in water, water vapor permeability and biodegradation data for the four formulations.
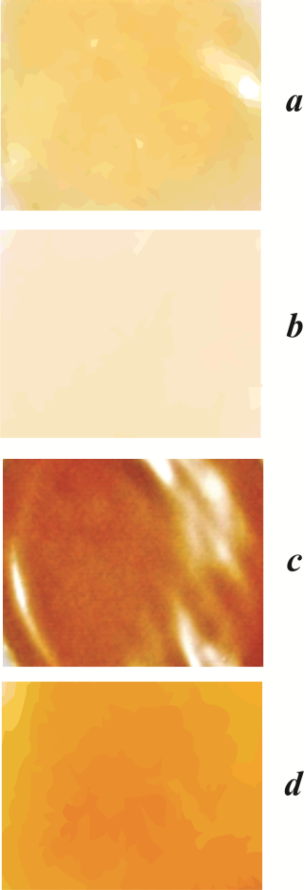 | Figure 2. Visual aspects of the four pectin biofilms with added babassu mesocarp and whey protein concentrate. (a) 100%P, (b), 50%P+50%WPC, (c) 50%P+50%BM (F3) and (d) 50%P+25%BM+25%WPC |
|
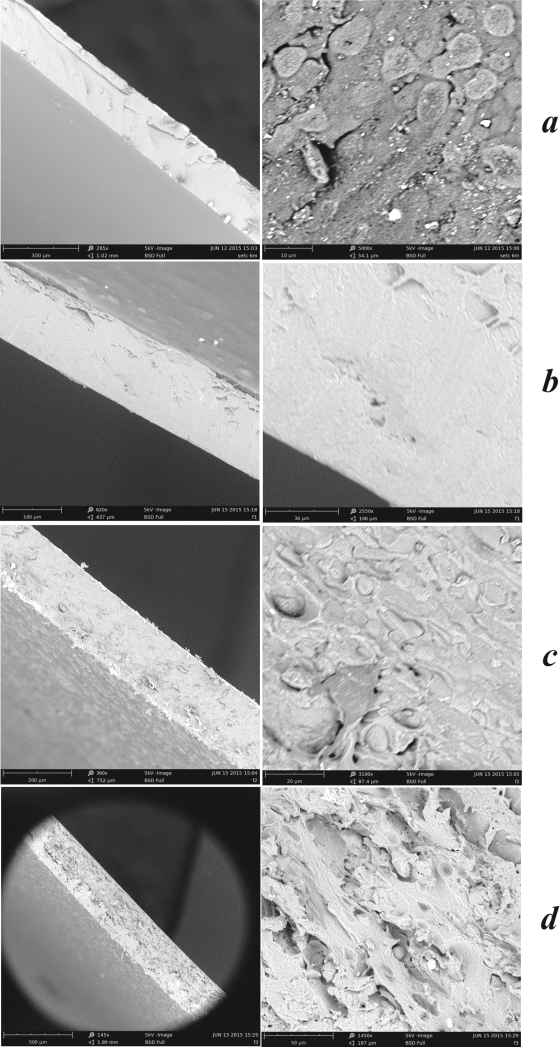
3.2. Morphology Results
- Figure 3 shows the SEM images for the formulations 100%P, 50%P+50%WPC, 50%P+50%BM and 50%P+25%BM+25%WPC, respectively.
3.3. Fourier-transform Infrared Spectroscopy (FTIR)
- The FTIR-ATR spectra obtained for the different biofilms can be seen in Figure 4. The characteristic pectin peaks can be observed at around 3400, 2900, 1700, and 1050 cm-1, attributed to the O-H, COO- (asymmetric), COO- (symmetric) and C-O-C stretching of the biopolymers, respectively. The peaks in the 1700 – 500 cm-1 region corresponded to several vibrations of the carbohydrate ring [34].
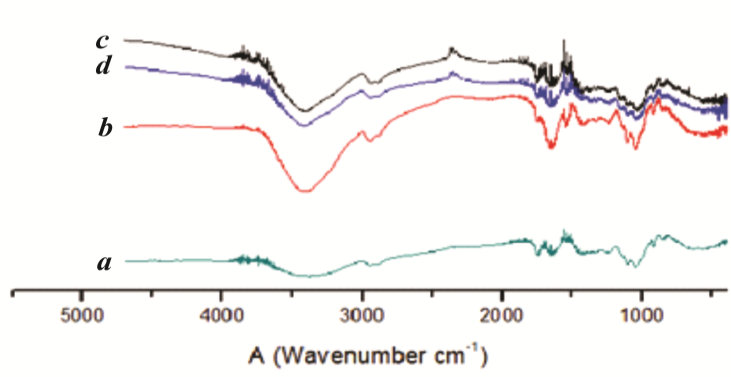 | Figure 4. FTIR-ATR spectra of the four biofilm formulations. (a) 100%P, (b), 50%P+50%WPC, (c) 50%P+50%BM (F3) and (d) 50%P+25%BM+25%WPC |
4. Conclusions
- Biofilms prepared by blending pectin with babassu mesocarp and whey protein concentrate presented improved properties when compared to the films prepared from the pure polymers. The composite biofilms composed of 100%P and 50%P+50%WPC showed translucent and homogeneous appearances. Moreover, the biofilms prepared with 50%P+50%BM and 50%P+25%BM+25%WPC presented similar structures, confirming the strong presence of BM in the formulation, making a stronger and more compact biofilm. In the present study a filmogenic solution composed of 50%P+50%BM with the addition of glycerol (3%, v/v) and CaCl2 (1% m/v) showed the best characteristics amongst the four biofilm formulations.
ACKNOWLEDGEMENTS
- The authors are grateful to the central analytical laboratory of CCET/UFMA and to Professors Arão Pereira da Costa Filho and Maria da Gloria Almeida Bandeira for offering their laboratories for the experimental procedures.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML