-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2017; 7(3): 59-63
doi:10.5923/j.materials.20170703.03

Effect of Various Dopant HCL Concentration on Electrical Conductivity of Pani-Cellulose Composite with Cellulose Isolated from Reed Plant (Imperatacy lindrica (L.))
Lela Mukmilah Yuningsih, Dikdik Mulyadi, Ilham Aripandi
Department of Chemistry, Muhammadiyah University of Sukabumi, Sukabumi, Indonesia
Correspondence to: Lela Mukmilah Yuningsih, Department of Chemistry, Muhammadiyah University of Sukabumi, Sukabumi, Indonesia.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

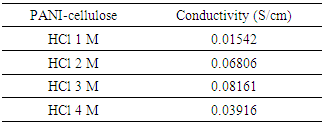
Material of polyaniline conductive were synthesized by chemical oxidative polymerization of aniline with cellulose isolated from freed plant with various concentration of HCl 1 M, 2 M, 3 M, and 4 M. Matrix cellulose isolated from reed plant by delignification method. Chemical oxidative polymerization of aniline was carried out by using K2Cr2O7 as initiator. Effect of various dopant HCl concentration on electrical conductivity of PANI-Cellulose composite was observed. The PANI-cellulose composite with dopant HCl concentration 3 M have the highest electrical conductivity 0,0816 S/cm. Analysis of surface morphology of composite using SEM-EDX showed that polyaniline particle dispersed on cellulose surface.
Keywords: Composite, Cellulose, Polyaniline, Conductivity, Dopant
Cite this paper: Lela Mukmilah Yuningsih, Dikdik Mulyadi, Ilham Aripandi, Effect of Various Dopant HCL Concentration on Electrical Conductivity of Pani-Cellulose Composite with Cellulose Isolated from Reed Plant (Imperatacy lindrica (L.)), American Journal of Materials Science, Vol. 7 No. 3, 2017, pp. 59-63. doi: 10.5923/j.materials.20170703.03.
Article Outline
1. Introduction
- Researching of developing conductive materials keep improving up to now. The development of conductive material now has been changed from inorganic compound to organic compound material. The most investigated conductive polymer is polyaniline (PANI), also known as aniline black. PANI has many advantages, such as ease of synthesis, low cost, good environmental stability, and the ability to be electrically switched between its conductive and resistive states [6]. Also, its wide applications in microelectronic devices, diodes, light weight batteries, sensors, super capacitors, microwaveabsorption, corrosion inhibition [17].PANI exists in various forms based on its oxidation level: the fully oxidized pernigraniline base, half-oxidized emeraldine base and fully reduced leucoemeraldine base (fig 1). On these PANI, emaraldine, is the most stable and conductive. Emeraldine base (EB) which has electrical insulator properties can be converted into emeraldine salt (ES) owns conductive properties by doping EB with protonic acid [10].
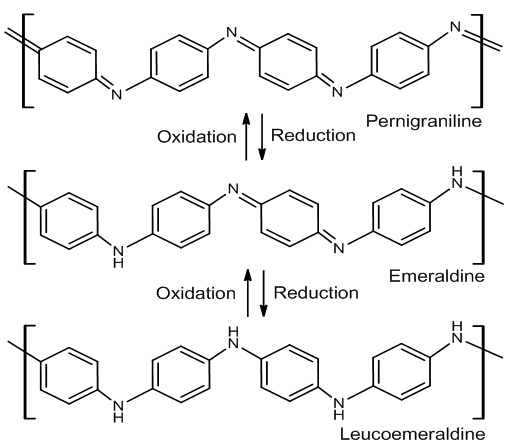 | Figure 1. Three forms of polyaniline bases [10] |
2. Experimentals
2.1. Material
- Reed plant, aniline (C6H5NH2), Sodium hydroxide (NaOH), Sodium hypochlorite (NaOCl), ethanol (CH3CH2OH), Technical acetone (CH3COCH3), Hydrochloric acid (HCl), and Potassium dichromate (K2Cr2O7).
2.2. Cellulose Isolation
- 16 g of reed powder was added 400 mL solution of NaOH 2%. The mixture was refluxed at 90°C for 3 hours. The bleaching step was soaking of sample for 1 hours in NaOCl 1% at 70°C [3]. The next step is purification, residue of delignification result is soaked in solution of NaOH 17,5% for 30 minutes. Moisture was filtered and washed with aquades and ethanol. Residue was dried in oven at 60°C.
2.3. Synthesis of PANI-cellulose Composite
- 0.5 g cellulose was added into 5 g aniline and stirred for 1 hours. The mixture of aniline-cellulose was dissolved into 75 mL HCl 1 M solution. Initiator solution was made of dissolving 7.89 g K2Cr2O7 into 75 mL HCl 1 M solution. The two solution were mixed at room temperature. Treatment of the same procedure to concentration of HCl 2 M, 3 M, and 4 M. The result of polymerization was filtered and washed with acetone. Than dried in oven at 60°C.
3. Result and Discussion
3.1. FTIR and XRD Analysis
- Fig 3a. shows the IR spectra of cellulose and PANI-cellulose composite. In a spectrum the band observed at 3444.08 cm-1 is due to –OH stretching and that one at 2902.58 cm-1 to the –CH stretching [13]. The peaks at 1649,23 cm-1 could correspond to the Alkyl group (C−C). The absorption band at 1277,7 cm-1 relates to C−O antisymmetric bridge stretching. The C−O−C pyranose ring vibration occurs in the region 1060,42 cm-1 [13]. The peak at 895,27cm-1 is originated from β-glycosidic linkages between glucose units in cellulose [11].The corresponding peaks for the polyaniline salt appear at 1560, 1482, 1306, 1245.9, 1148 and 814 respectively [17]. Fig. 3, The characteristic broad peaks at 3423.37 cm-1 could be assigned to –OH and N−H stretching vibration of composite compounds [5]. The bands at 1560.07 cm-1 and 1479.39 cm-1 can be attributed to C=N stretching vibration of quinoid ring C=C stretching vibration of benzenoid ring modes, respectively [8]. The peaks at 1301.06 cm-1 and 1243.95 cm-1 correspond to N-H bending and the symmetric component of the C-C (or C-N) stretching modes in QBQ, QBB and BBQ [2]. The bands at 1142.91 cm-1, assigned to a vibration mode of conducting protonated (−NH●+=) structure in the spectrum of PANI hydrochloride is overlapped by the strong absorption bands of cellulose (Fig. 3a). The band of outof-plane deformation of C−H on a 1,4-disubstituted ring at 803.73 cm-1 is also well recognizable in the spectrum of the covered fibers [9].The results indicate that different functional groups are involved in the dye adsorption and that certain bonds were formed between the Ce/Pn composite and dye molecule which caused the changes in vibration frequency of these chemical groups. The results are consistent with previous studies reporting the involvement various functional group in the adsorption of dyes onto polyaniline composites [15].The XRD pattern of the synthesized PANI-cellulose composite is shown in Fig. 3b. The diffraction peaks at 2θ = 20.42 and 25.04° were assigned to characteristic semi-crystallin structure in polyaniline. The peaks 2θ = 25.04° is corresponding to the periodicity parallel and perpendicular to polyaniline chains [12]. The broadening of diffraction in 24°-28° shows polyaniline nanocrystal structure. The cellulose structure exhibited characteristic peaks at 2θ = 14,55 and 21,74° [7]. The degree of crystallinity PANI-cellulose composite obtained 15,52%.
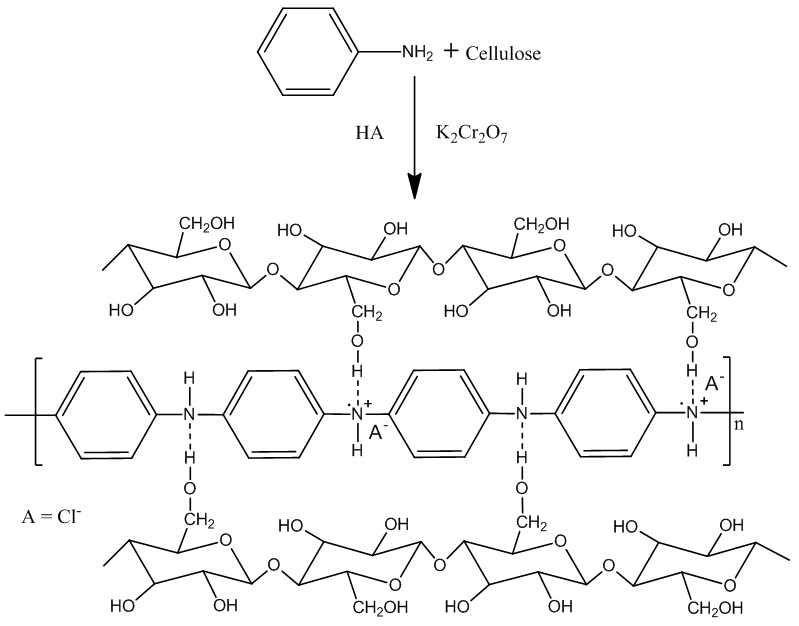 | Figure 2. Reaction of composite formation [15] |
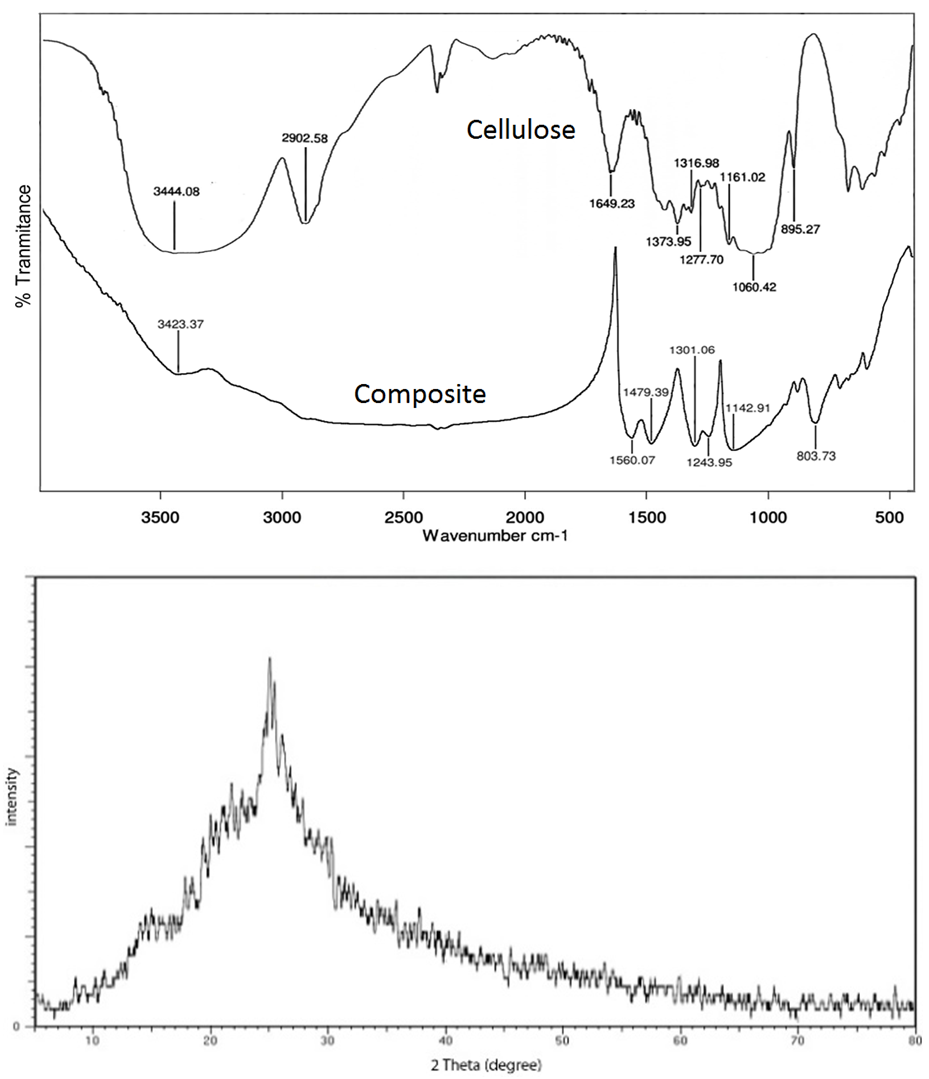 | Figure 3. FTIR Spectra of reed plant cellulose, PANI-cellulose composite by HCl 3 M (a), and diffractogram of PANI-cellulose composite by HCl 3 M (b) |
3.2. Conductivity
- Polyaniline can be doped to the highly conducting is called “emeraldine.” It consists of amine (–NH–) and imine (=N–) sites in equal proportions. The imine sites are protonated by acids HA to the bipolaron (dication salt) form [16]. The structures at each stage of this process are shown in Figure 4. However, this undergoes a further rearrangement to form the delocalized polaron lattice, which is a polysemiquinone radical-cation salt. Polarons are the charge carriers responsible for the high conductivity in polyaniline. The presence of coulombic interactions dielectric screening and local disorder in the polyaniline lattice act to stabilize the delocalized polaron state.
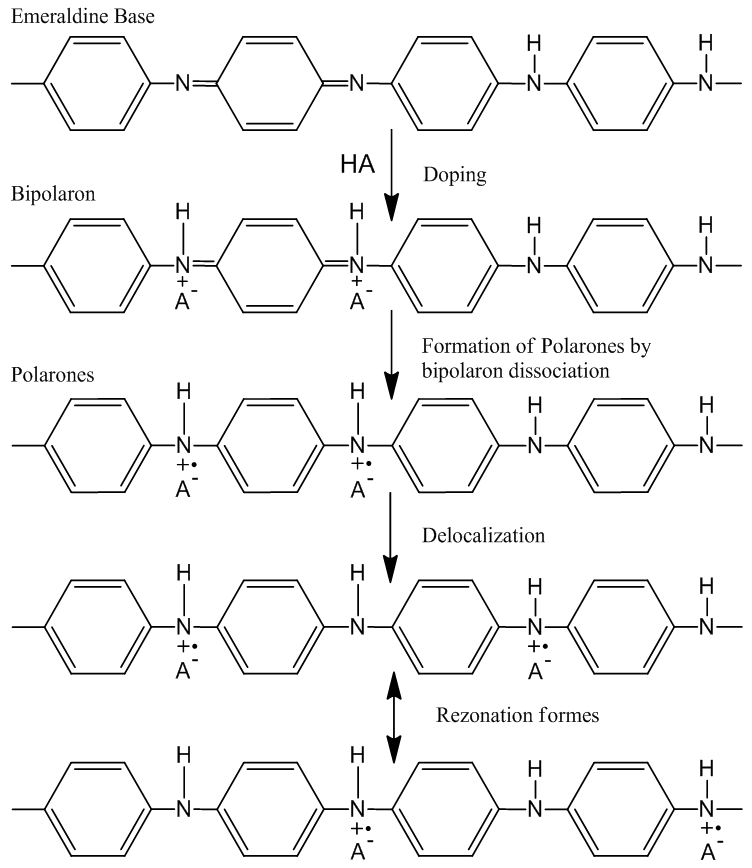 | Figure 4. The doping of EB with protons to form the conducting emeraldine salt form of polyaniline (a polaron lattice) |
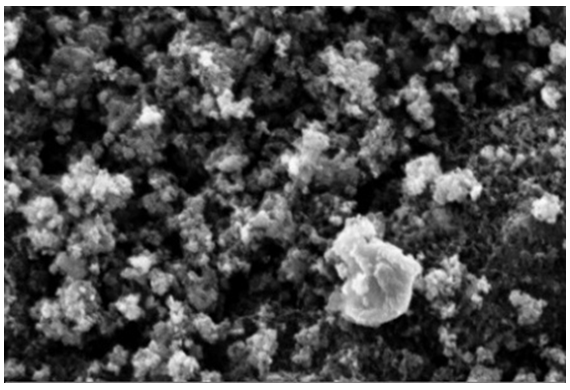 | Figure 5. Surface morphology of PANI-selulosa composite by HCl 3 M |
|
3.3. SEM-EDX Analysis
- Result of characteristic PANI-cellulose composite with Scanning Electron Microscope (SEM) showing covered cellulose surface by granular shaped particles. This shows that polyaniline was attached very well on each cellulose fiber. Related to high surface area of cellulose, this composite could intensify the conductivity. The composite has the characteristic features of both polyaniline and cellulose. The chemical composition of the PANI-cellulose composite with selective area analysis was done by energy dispersive X-Ray spectroscopy.HCl doped PANI has structure of aggregates from particle and dispersed on cellulose fiber. Analyze result can be observed on table 2.
|
4. Conclusions
- PANI-cellulose composite were successfully synthesized by the chemical oxidation polymerization process. The structure of composite and its composites have been analyzed by FTIR spectra and have a strong interaction between PANI and cellulose fiber. Conductivity of PANI-cellulose composite effected most by level of dopant concentration. The optimum conductivity is the composite with HCl 3 M. Analyze of surface morphology shows that polyaniline forms aggregate on cellulose fiber.
ACKNOWLEDGEMENTS
- The authors wish to thank RISTEKDIKTI for the financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML