-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2016; 6(6): 152-156
doi:10.5923/j.materials.20160606.02

Development of Gypsum-Carbonated Apatite Biphasic Granules for Paediatric Orthopaedic Application
Syazana Abu Bakar1, Nurul Awanis Johan1, Siti Farhana Hisham1, Mohamad Azmirruddin Ahmad1, Najihah Ismail1, Abdul Yazid Abdul Manaf1, Siti Noorzidah Mohd Sabri2, Shirin Ibrahim1
1Advanced Material Research Centre (AMREC), SIRIM Berhad, Malaysia
2Rock Based Technology Section, Mineral Research Centre
Correspondence to: Syazana Abu Bakar, Advanced Material Research Centre (AMREC), SIRIM Berhad, Malaysia.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The main discovered material with closer chemical composition to the bone mineral is known as carbonated apatite. This material predicted to be a good artificial bone substitute with good resorption and bioactivity properties.Main focus of this study is on developing suitable bone substitute by fabricating biphasic gypsum-carbonated apatite granules. Gypsum which is known as calcium sulphate dihydrate (CSD) granules was immersed into the 1 M of carbonate and phosphate salt solution at 80°C for 24 hours treatment time. The development of these biphasic granules were studied using X-ray diffraction (XRD), Fourier transform infrared (FTIR) and Scanning Electron Microscopy (SEM). The compositional elucidation was quantitatively measured by CHN analysis to obtain the contents of carbonate, CO3. The ions release study was conducted in simulated body fluids (SBF) and quantitated using Atomic Absorption Spectroscopy (AAS) to get the total release of calcium ions upon immersion process. The obtained results are shows that the CSD was successfully transformed into carbonated apatite layer to form biphasic granules.
Keywords: Carbonate apatite, Phase transformation, Biphasic granule, Bone substitute
Cite this paper: Syazana Abu Bakar, Nurul Awanis Johan, Siti Farhana Hisham, Mohamad Azmirruddin Ahmad, Najihah Ismail, Abdul Yazid Abdul Manaf, Siti Noorzidah Mohd Sabri, Shirin Ibrahim, Development of Gypsum-Carbonated Apatite Biphasic Granules for Paediatric Orthopaedic Application, American Journal of Materials Science, Vol. 6 No. 6, 2016, pp. 152-156. doi: 10.5923/j.materials.20160606.02.
Article Outline
1. Introduction
- Bones can be defected usually from certain disease, tumor resection, surgery, trauma, fractures, congenital malformation and also periodontitis in dentistry [1]. In order to cure this defection, the reconstruction process has to be done by selecting the suitable method includes of auto graft, allograft, demineralized bone matrix, hydroxyapatite calcium phosphate (CP, TCP), autologous bone marrow aspirates, bone morphogenetic proteins and other methods that related to the growth factors. Special properties that must be featured in the synthetics bone substitute are biocompatible, show minimal fibrotic reaction, undergo remodeling and support new bone formation in order to reconstruct the defections [2].Ceramic is one of material to be widely used in reconstructing defected bones and many studies had reported on the ideal properties that suitable as bone substitute. Researchers had considered many aspects of this kind of materials to be a good bone substitute based on their chemical composition which influence the rate of solubilisation, resorption and also the bioactivity [3]. Based on the resorbable properties, the best option to be chosen as bone substitute is Hydroxyapatites (HA), Calcium sulphate hemihydrate (CSH), calcium sulphate dehydrate (CSD) and other calcium phosphates (CP). HA is a well-known bioceramic material to be used in bone reconstruction process, however the slow resorption and unpredictable resorption rate of this material is not really useful for bone reconstruction process in paediatric orthopaedic application [2]. Previous study has reported that the rate of resorption can be increased by ionic substitution of some salts such as carbonate and phosphate ions in order to form the apatite layer [3]. A lot of research works had done in order to find the promising material for bone replacement. A dominant inorganic component was founded in a bone phase is known as carbonate apatite (CO3Ap) [4]. This material is a best candidate to be bioceramic bone substitute in replacing new bone based on the remodeling process [5]. The main focus for this work is developing the biphasic carbonated apatite to be desired bone substitute for children bone reconstruction.CO3Ap with the specialty of 4 to 8 wt. % of carbonate contents in apatitic structure can be resorbed by the osteoclasts which replaced the new bone. This artificial bone substitute is commonly produced in powder forms in order to ease the fabrication process. However, these powdery forms lead to the inflammatory reaction effect when powder is exposed to the soft tissue. Recent work has been done by fabricating the granular forms of CO3Ap by thermodynamically for various sizes in certain time exposure. Current preparation on the biphasic granules were conducted using common method which is known as dissolution and precipitation method for this study. The calcium sulphate dihydrate (CSD) was treated in carbonate and phosphate salt solution under 80°C for 24 hours. Calcium sulphate dihydrate (CSD) is a chosen starting material; it has ability in promoting the bone regeneration because of their excellent osteoconductivity and free molded properties [6]. The XRD analysis was done to confirm the formation of gypsum and hydroxyapatite phases in the biphasic granules. The FTIR spectroscopy specialized in indicating the formation of carbonate apatite that formed in the biphasic granules. The cross sectional morphology and also phase confirmation of the biphasic granules was observed using SEM. The significant composition of carbonated apatite granules was determined by CHN analysis. The total release of calcium ions upon immersion in simulated body fluids (SBF) at 37°C for certain interval time includes of day 7, 21, 28 and 35 was examined by AAS analysis.
2. Details Experimental
2.1. Materials and Method
- The preparation of sample were started by mixing process of CSH powders with distilled water with water to powder ratio of 0.5 to make a paste. The paste was then packed into mold to produce the calcium sulphate dihydrate (CaSO4. 2H2O, gypsum). Different sizes of gypsum granules were produced through the crushed and sieve method into certain sizes includes of 300-600, 600 – 1000 and 1000 - 2000 µm. The gypsum granules were then being immersed into the carbonate and phosphate salt solution at 80°C for 24 hours. 1M NaHCO3 and Na2HPO4 were prepared as reactants introducing CO32- and PO42- groups. The resultant precipitates after washing with deionized water were dried at 60°C and continuously overnight drying at 40°C. The fabricated granules were then analyzed through physical and chemical analysis. The samples were characterized by XRD, FTIR, SEM, CHN and AAS analysis. For XRD analysis, a Bruker D8 Advance X-ray diffractometer was used to elucidate the phase transformation. For FTIR analysis, the FTIR spectrometer (Perkin Elmer) was used to analyze the existing of functional group in the sample and spectra was acquired at room temperature using KBr disk method. The morphological structures of sample were observed under SEM (LEO 1525) through the cross sectional of sample. The carbonate contents were quantitatively examined through the CHN analysis. The quantitative determination of calcium ions concentration was investigated using AAS to get the total concentration of released calcium ions upon immersion in simulated body fluids (SBF) at 37°C for certain interval time includes of day 7, 21, 28 and 35.
3. Results and Discussion
3.1. Phase Transformation Using XRD Analysis
- Phase transformation was confirmed through the XRD analysis; there is pattern of samples that treated in carbonation and phosphorization process at 80°C upon immersion for 24 hours. It was observed that the gypsum phase of granules started to transform into apatite phase after 24 hours. Based on Figure 1, there are broad peaks appears at 2 theta = 32° indicating the formation of amorphous apatite layer after 24 hours.
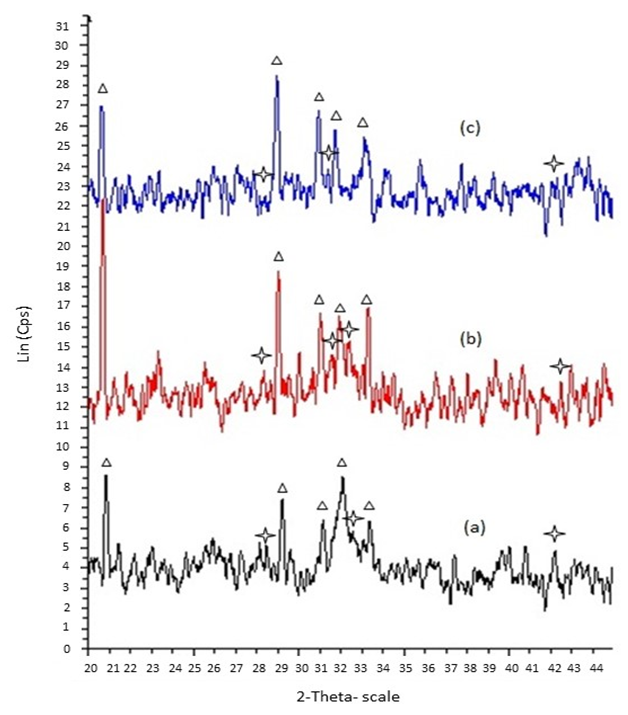 | Figure 1. XRD patterns of gypsum granules and biphasic granules at various times (a) 300- 600, (b) 600 – 1000, (c) 1000 – 2000 µm; Apatite, Gypsum |
3.2. Carbonated Apatite Determination through FTIR Analysis
- The existence of carbonate group is clearly determined in the FTIR spectra, by comparing the starting material spectra and the solely hydroxyapatite spectra with the other treated biphasic granules. Various sizes of granules were hydrothermally treated for 24 hours through dissolution and precipitation method. Previous findings reported that the carbonated ion substitution in hydroxyapatite can be classified into different types includes of A type, B type and AB type. From the spectra for biphasic granules, the absorption bands at 1471.2 and 1415 cm-1 represented the doublets peak of CO3 group which is classified as an apatite of B type [4, 5, 7]. As seen in Figure 2, other spectra were detected from the granules where the P-O band falls within range 873 and 900 - 1300 cm-1 is assigned as PO4 group [7] whilst the SO4 group at 1113 - 1119 cm-1. The absorption bands for CO3 and PO4 group appeared to be the apatite phase when the phosphate ions, PO42- was partially substituted by carbonate ions, CO32- in the apatite lattice. Remaining gypsum contents that existed in the biphasic granules caused the absorption of SO4 band in the spectra.
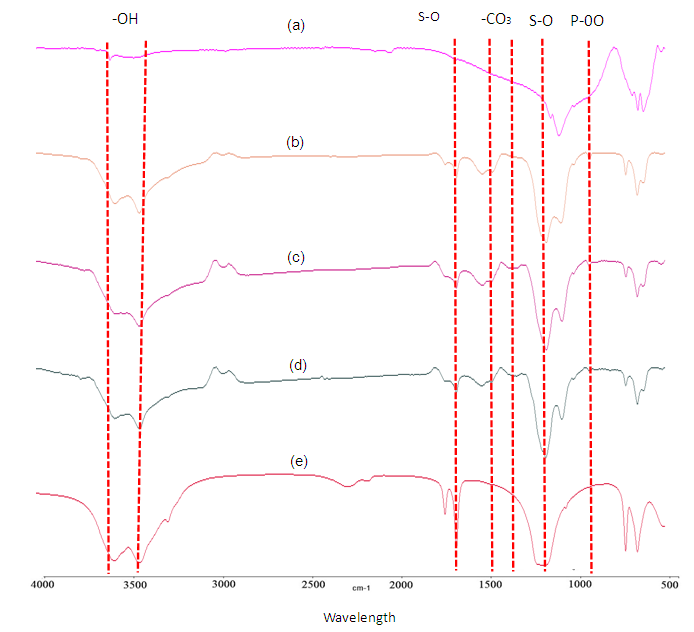 | Figure 2. FTIR spectra of gypsum- CO3 Ap granules prepare using calcite base for 24 hours; (a) hydroxyapatite (b) 300-600 (c) 600-1000 (d) 1000-2000 µm and (e) gypsum |
3.3. Microstructure Observation of Biphasic Granules
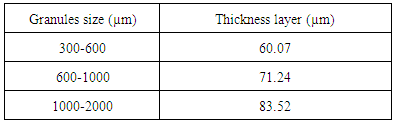
- Referred to the images 1(a), 1(b) and 1(c) are the SEM micrographs of the cross sections of the biphasic granules for various granules size which is 300-600, 600-1000 and 1000-2000 µm. From the micrographs, the existing of two different phases was seen as the outer layer is corresponding to the apatite phase whilst the central part contains of porous section. Focus micrograph for the porous microstructure were observed as in micrograph 2 (a), 2 (b) and 2 (c) which is represented for gypsum phase. The micrographs revealed that the formed apatite phases showed dense structure rather than the gypsum part in the granules. It is clearly shown apatite was formed by the successive of the carbonation and phosphorization process in this work. The dissolution process of the outer layer of the granules were released the ions that reacted with the salt solution. The ionic reaction between those ions implying to the deposition process of carbonated apatite layer onto the granules surface. As the granules size increased, the formed apatite layer will also become thicker. It is measured that the thickness of apatite layer gradually increased from range 60.07 µm up to 83.52 µm as seen in Table 1. The porous structured of gypsum phase has their own specialty in giving certain properties for the bioactivities to reconstruct and formation of new bone. P. V. Giannoudis et al. discovered that formed porosity will allows the phagocytic action, resorption and filtration by bone-forming cells, nutrients and also growth factors for the bone recovery [9]. These studies were also supported from the previous finding where the existence of different phases in granules, refer to the gypsum-carbonated phases proved in enhancing the osteogenesis activity [10].
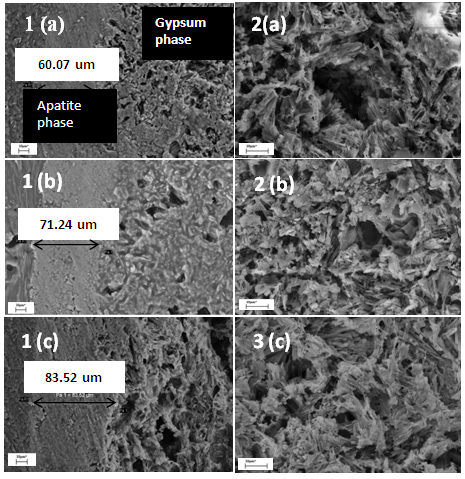 | Figure 3. The cross sectional images (1) and porosity structures (2) of gypsum – CO3Ap granules prepare using gypsum base with different granules size; (a) 300-600, (b) 600-1000 and (c) 1000-2000 µm |
|
3.4. Carbonate Contents of the Biphasic Granules
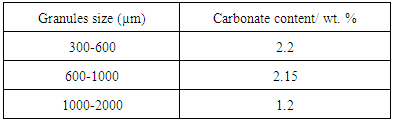
- The result obtained from the XRD pattern, FITR spectra and also the SEM micrographs clearly demonstrated that there is formation of carbonated apatite in these granules. The results were strengthening through the CHN analysis as shown in Table 2. The existence of carbonate element was detected in the samples for each granular size. As indicated by the FTIR results, these biphasic granules is classified in B type carbonated apatite where the PO4 were partially substituted by CO3 that is containing 1 – 2 wt. % of carbonate. It is observed that all granules size has carbonate contents for each sizes and discovered that the combination of different size of granules will give a certain amount of carbonate. This analysis confirmed that this biphasic gypsum-carbonate apatite granules have the ability to act as good as the human bone.
|
3.5. Concentration Determination of Calcium Ions from Biphasic Granules
- The release study for these biphasic granules involves the SBF as buffer, which contains of ions rich simulated body fluid (SBF) that is similarly to the human plasma. This work is undergone for several interval times starting from first week goes to the 35 days. The buffer solutions were taken during the immersion process to determine the changes in concentrations of calcium released. The correlation between reactions of biphasic granules with the buffer was observed to confirm whether this material is potential to be bone substitute. The concentrations of ion release were measured through the AAS analysis in order to get the release pattern of the biphasic granules. This analysis involves the dissolution of Ca2+ ions by exposing the gypsum in simulated body fluid (SBF) solution. As seen in Graph 1, the release of calcium ions were observed upon immersion from the first day up to day 35 which indicates the release is high as early as day 7. The reductions of release were slightly shown in the graph to the constant rate from day 21 to day 35. It is expected that this biphasic granules were reacted with the solution and slowly release the Ca2+ [6, 10]. The release ions allowed the precipitation and deposition of carbonated apatite, where the Ca2+ reacted with the available minerals and ions in the solution in order to reconstruct the defected bones. From the findings, it is suggested that the biphasic granules can be a good bone substitute when it can be similarly mimicking the actual bone mineral. The constant release of Ca2+ proved that the materials can supply the calcium sources for the deposition process for new bone formation. Depositions of Ca2+ onto the bone were expected, which is gives more absorption properties to form the new bone growth.
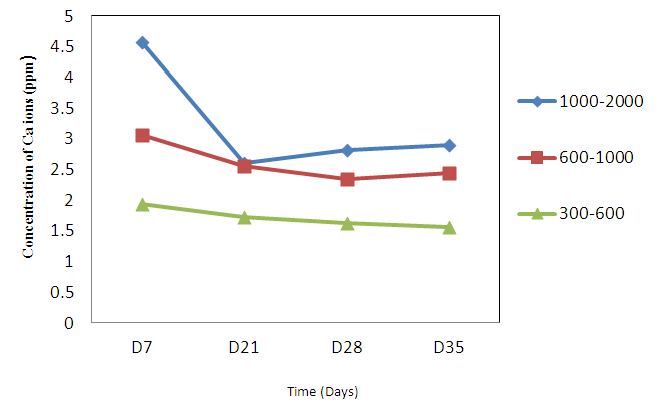 | Graph 1. AAS results for Calcium ions release of biphasic granules |
4. Conclusions
- Development and characterization of the fabricated biphasic granules of gypsum-carbonated apatite with different size is succeed. This ceramic material shows a good properties for the adaptation with human bones. The formation of apatite layer was successfully done through dissolution and precipitation methods by exposing the gypsum granules to the salt solution. It is proven that the apatite layer was formed at the outer layer of granules whereas the core side of granules still in gypsum phase. Various sizes of granules is not particularly gives significant changes in their properties. Data analyses supported this material is highly potential to be a bone substitute. In conclusion, the biphasic granules of gypsum-carbonated apatite might be useful to be a promising material in bone reconstruction application.
ACKNOWLEDGMENTS
- The authors are gratefully acknowledged the supported by Ministry of Science, Technology and Innovation (MOSTI) for support in providing their research grant.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML