-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2016; 6(5): 115-124
doi:10.5923/j.materials.20160605.01

Adsorptive Removal of Lead Ions from Aqueous Solution Using Biosorbent and Carbon Nanotubes
Ahmed A. Moosa, Ali Mousa Ridha, Noor Amir Hussien
Department of Materials Engineering Technology, Engineering Technical College– Baghdad, Middle Technical, University Baghdad, Iraq
Correspondence to: Noor Amir Hussien, Department of Materials Engineering Technology, Engineering Technical College– Baghdad, Middle Technical, University Baghdad, Iraq.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

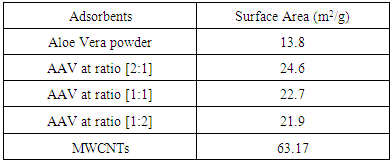
In this work, the removal of poisonous Pb (II) from aqueous solution by different adsorbents was investigated. Aloe Vera plant (AV), activated Aloe Vera leaves powder (AAV) and multi walled carbon nanotubes (MWCNTs) were used as different adsorbents. The modification of Aloe Vera plants was carried out by acid activation. The adsorption process was characterized by FTIR and BET. The results of (BET) showed improvement in surface area of Aloe Vera (AV) after activation from (13.8 m2/g) to (24.6 m2/g). The results of (FT-IR) exhibited new the functional groups such as (C=C, C-Hs, C-Os) of acid activated Aloe Vera. Also the results of (BET) showed MWCNTs have (63.9 m2/g) surface area. The results of (FT-IR) showed MWCNTs have functional groups are (O-H, C=H, C=C). The effect of different parameters such as adsorbent dosage, pH of adsorbate solution and contact time was studied for removing of pb2+ from aqueous solutions onto each of adsorbent of AV, AAV and MWCNTs. The maximum adsorption efficiency of pb2+ onto each of adsorbents at best conditions were selected to be (97.29%) for AV at (2.2 g, pH 5and 6 hr), (97.28%) for AAV at (1.6 g, pH 5 and 6hr) and for MWCNTs are (97.55%) at (0.06 g, pH 5 and 6hr). The equilibrium data characterized by the models. The Freundlich isotherm correlates well with the experimental data than Langmuir and Temkin adsorption isotherm when using AV, AAV and MWCNTs as adsorbent.
Keywords: Adsorption, Heavy metals, Isotherm, Bioadsorption, Carbon Nanotube
Cite this paper: Ahmed A. Moosa, Ali Mousa Ridha, Noor Amir Hussien, Adsorptive Removal of Lead Ions from Aqueous Solution Using Biosorbent and Carbon Nanotubes, American Journal of Materials Science, Vol. 6 No. 5, 2016, pp. 115-124. doi: 10.5923/j.materials.20160605.01.
Article Outline
1. Introduction
- Heavy metals are conventionally defined as metallic elements an atomic number >20. The most common heavy metal contaminants are Cd, Cr, Cu, Hg, Pb, and Zn. It was reported by many authors that heavy metals is a term which applies to the group of metals and metalloids with atomic density greater than 4 g/cm3, or 5 times or more, greater than water (Duruibe et al. 2007).Lead is one the major metal ions hazardous to the human body through inhalation, skin contact or with diet, and can produce adverse effects on virtually every system in the body. Low levels of Pb(II) have been identified with anemia while high levels cause severe dysfunction of the kidneys, liver, the central and peripheral nervous system (Eren et al. 2009). The maximum concentration in drinking water standards identified by 0.05 mg. L−1 for lead (Bala et al. 2008).Several treatment processes are used For removing heavy metals from wastewater include reduction, precipitation, ion exchange, electrochemical reduction, and reverse osmosis (Annadurai et al. 2012). These processes are expensive, not eco-friendly, high power requirement, incomplete metal removal (Sabat et al 2012).Adsorption technique is successively alternative process that utilized for removing heavy metals from industrial wastewater, which can be performed in batch mode or continuous process. Adsorption processes have offered flexibility in design and operation and in many cases will produce high-quality treated effluent. Also adsorption is sometimes reversible process, adsorbents can be reformed by the suitable desorption process therefore adsorption mostly method applied to remove metals (Fu and Wang 2011).Biosorption which applies biological materials, thus process can considered as a relatively modern technology for removing even trace concentrations of heavy metals from wastewater (Matei et al. 2015).Agricultural and plant waste based by-products have good demonstrated bio-sorption potential for heavy metal ions (Gupta 2015).CNTs as a good adsorbents have attracted increasing attention of many researchers because of their highly porous and hollow structure, large specific surface areas, capable of π-π electrostatic interaction, light density and strong interaction between CNTs and heavy metal ions and organic compounds (Ren et al 2010), (Chen et al 2009) and (Augusto 2010).The aim of this work is to study the removal of Pb+2 from wastewater using different adsorbents: Aloe Vera (AV), activated Aloe Vera (AAV) and multi walled Carbon nanotube (MWCNTs) and compare their adsorption efficiency. The influence of adsorbent dosage, pH and contact time on the adsorption efficiency of Pb+2 were investigated to obtain the better operating conditions of adsorption process. The adsorption isotherm models, namely Langmuir, Freundlich and Temkin were studied to describe the adsorption equilibrium.
2. Materials and Methods
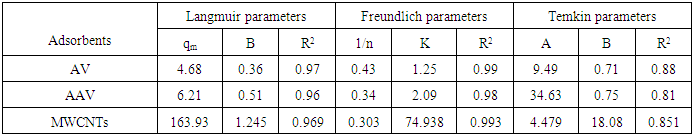
- 1) AdsorbateLead Nitrate salt Pb(NO3)2 was used to prepare 1000 mg/L standard stock solution of Pb (II) solution. A 50 ml of distilled water was added to 50 ml of HNO3 in a 100 ml beaker and stirred using a magnetic stirrer. Distilled water was then added to get 1000 ml of acidic solution of lead nitrate with stirring. This acidic solution was added to 1.78gm of Pb (NO3)2. Distilled water was then added to get 1000 ml of acidic solution of lead ions with stirring. Atomic absorption spectrometer showed that the concentration of lead ion solution is 1000 ppm. The pH of the lead ions initial concentration was adjusted to a certain pH by adding NaOH or HCl.2) AdsorbentsA) Aloe Vera PlantFresh green Aloe Vera leaves were collected from local gardens. The collected leaves were washed extensively with distiller water for several times to remove dust. These leaves were cut into equal sized pieces and allowed to dry in the sun for 5 days so as to remove the water and the keeping of the Aloe Vera components. Then the leaves were dried in an oven at 50°C for 2 hours. The dried Aloe Vera leaves were then ground using a home electrical grinder into fine powder. Finally, the bioadsorbent powder was sieved to obtain [75-125μ] particle sizes and kept in an airtight container.A.1) Acid Activation of Aloe Vera Powder (AAV)Aloe Vera powder was acid activation by using [HNO3: H2SO4] at different ratio [2:1], [1:2] and [1:1]. 1 g of Aloe Vera powder was added to 20 ml of acid solution and then mixed using a magnetic stirrer for 6 hrs at 120 rpm. After that Aloe Vera acid solution was left for 24 hours at room temperature followed by washing several times with distal water until pH reached a constant value. After this processing, the modified Aloe Vera was dried in a furnace at 80°C for 6 hrs and the acquired acid activation Aloe Vera (AAV). B) Multi Walled Carbon Nanotube (MWCNTs)High Purity 99%, Multi Wall Carbon Nanotubes (MWCNTs), Intelligent Materials Pvt. Ltd., Punjab, India will be used as an adsorbent. MWCNTs have length 10-20 nm and a diameter between 3-8 µm. B.1) Functionalization of MWCNTsCNTs are materials practically insoluble, or difficult to disperse, in any kind of solvent. Chemical functionalization is used to attache different functional groups on the side wall or end caps of nanotubes without significantly changing their desirable properties (Dobrzański et al. 2010). 0.5 g of MWCNTs was immersed in 500 ml of mixture of concentrated (HNO3:H2SO4) (1:3 by volume) at room temperature. The MWCNTs solution with acid was ultrasonicated using ultrasonic path at (frequency 60 KHz, power 140 W) for 40 min at 40°C. The solution was then diluted 15 times with deionized water and then filtered using vacuum filtration system with 0.2 μm pore size filter papers. This washing operation was repeated until the pH became the same as that of deionized water. The MWCNTs on the filter paper was then left to dry at room temperature for 12 hours. After drying a sharp blade is used to remove CNTs from the filter paper and then dried in an oven at 60°C for 6 hrs (Moosa et al. 2010) (Moosa et al. 2015).C) Characterization of AdsorbentsThe specific surface area of AV, AAV, and MWCNTs were measured using the BET analysers (model 9600, USA). AV, AAV and MWCNTs were characterized using the Fourier-Transformation Infrared Spectra (FT-IR) (IRTracer-100 / Shimadzu Co. / USA).D) Batch Adsorption SystemD.1) Adsorbent DosageThe best of adsorbent dosage for adsorption of Pb(II) ions onto Aloe Vera, acid treated Aloe Vera powder and MWCNTs were obtained experimentally. Different dosage (0.2, 0.4, 0.6, 0.8, 1, 1.2, 1.4, 1.6, 1.8, 2, 2.2, 2.4g) for Aloe Vera and activated Aloe Vera powder, (0.02, 0.04, 0.06, 0.08 and 0.1g) for MWCNTs was added to 50 mL with initial concentration of 100mg/L of pb2+ solution. The pH of each solution was adjusted to desired 5.45 using dilute of NaOH or HCl. The solution was then shaken for about 5hrs in a rotary shaker at fixed speed 125 rpm and temperature of 25°C. The samples were then filtered and the remaining concentration of pb2+ was measured by atomic-absorption spectrophotometer. The best dosage of AV, AAV and MWCNTs were chosen at maximum adsorption efficiency (maximum removal).D.2) Effect of pHIn order to obtain the optimum pH for adsorption of Pb(II) onto AV, AAV, and MWCNTs a series of batch experiments were conducted at various pH in the range of (1, 2, 3, 4, 5, 6 and 7) for Pb(II). The best dosage of AV, AAV and MWCNTs were added to 50 mL with initial concentration of 100mg/L of pb2+. The pH of solutions was adjusted to desired value using dilute of 1M NaOH or 1M HCl. The solution was placed in bottles and then agitated for about 5 hrs in a shaker at 125 rpm and 25°C. The samples were then filtered and the remaining concentration of Pb2+ was measured by atomic-absorption spectrophotometer. The best pH of lead solution was then obtained at maximum adsorption efficiency (maximum removal).D.3) Effect of Contact TimeThe effect of contact time on the adsorption of Pb(II) onto AV, AAV and MWCNTs was investigated using various adsorption times from 2hr to 10hr. The solutions of Pb+2 was adjusted to the best pH with the initial concentration 100 mg/L. A volume 50 mL of Pb(II) solutions was placed in the bottles. The best dosage of each AV, AAV and MWCNTs were added to each bottle. The solution in the bottles was agitated for different times in a shaker with water bath at 125 rpm and 25°C and then filtered. The equilibrium concentration of lead ions was determined by atomic-absorption spectrophotometer. The best time was then obtained at maximum adsorption efficiency (maximum removal).E) Equilibrium IsothermsThe equilibrium isotherms for adsorption of Pb+2 ions onto AV, AAV and MWCNTs were performed experiments using the best dosage for each adsorbents, the best pH of Pb+2 ions solutions and best adsorption period. The solutions of Pb+2 ions with different initial concentration (20, 40, 60, 80, 100, 150 and 200 mg/L) were adjusted to the best pH of Pb+2 ion solutions. A 50 mL of Pb+2 ion solutions was placed in the bottles. Best dosage of AV, AAV and MWCNTs were added to each the bottle. The samples in the bottles were agitated at 125 rpm and at 25°C. The samples were then filtered and the remaining concentration of lead ions was measured by atomic-absorption spectrophotometer.E.1) Langmuir Isotherm ModelThe Langmuir model considered the first theoretical of nonlinear sorption, which sorption occur on a homogenous surface without interaction between adsorbed molecules. These models depend on three conditions: adsorption process works on monolayer only, every surface of his ability on the adsorption equality and the ability of active side to adsorb was changed from one to another (Dada et al. 2012). The Langmuir isotherm equation is (Dada et al. 2012)
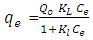 | (1) |
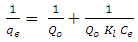 | (2) |
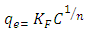 | (3) |
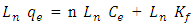 | (4) |
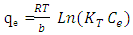 | (5) |
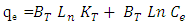 | (6) |
3. Results and Discussion
- A) FT-IRThe Fourier Transform Infrared Spectrophotometer, FTIR, spectra of Aloe Vera is shown in Figure 1. The presence of strong and broad intensity band around (3410.15 cm-1) was assigned to phenolic OH stretching (Ray and Gupta 2013) and (Ray et al. 2013). The peak at 2924.09 cm-1 corresponds to -CH stretching that might be due to H-bond formation at higher concentrations. The absorbance bands were seen in the range of 1732.08 cm-1 and 1269.16 cm−1 which attributed to C=O and C-O-C stretching of acetyl groups present in the sample (Kiran and Rao 2014). Moreover, the bands at 1650–1578 cm−1 implied the presence of C=O stretching and indicated the presence of carboxyl components and phenolic compounds which are polar in nature (Ray and Gupta. 2013) and (Ray et al. 2013). The absorbance spectrum at around 1732.08 cm−1, 1597.06 cm−1, and 1269 cm−1 can be accounted for the presence of C=O, COO-, and C-O-C stretches of acetyl groups (Hulle et al. 2014) and (Femenia et al. 2003). The CH3 and COO (corresponds to 1431.18 cm−1). The identification of strong bands in the range of 1076.26-1033.85 cm-1 due to the presence of polysaccharide sugars, such as galactose and glucans (Barandozi & Enferadi 2012).
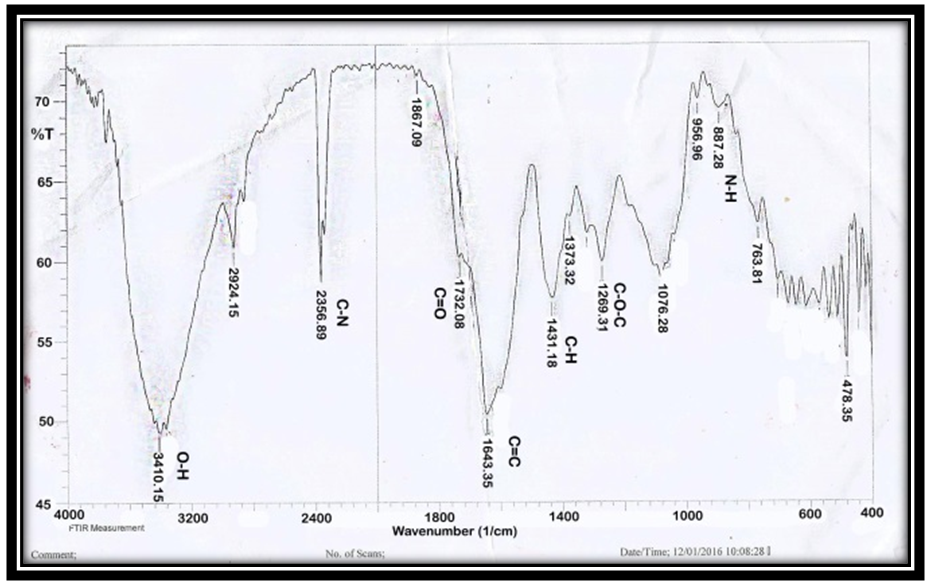 | Figure 1. FT-IR of Aloe Vera |
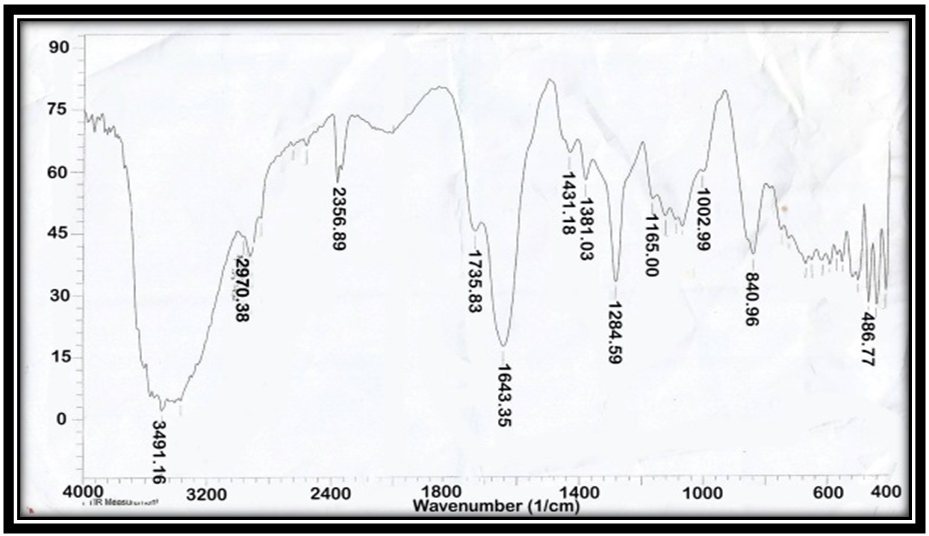 | Figure 2. FT-IR of AAV at [2:1] |
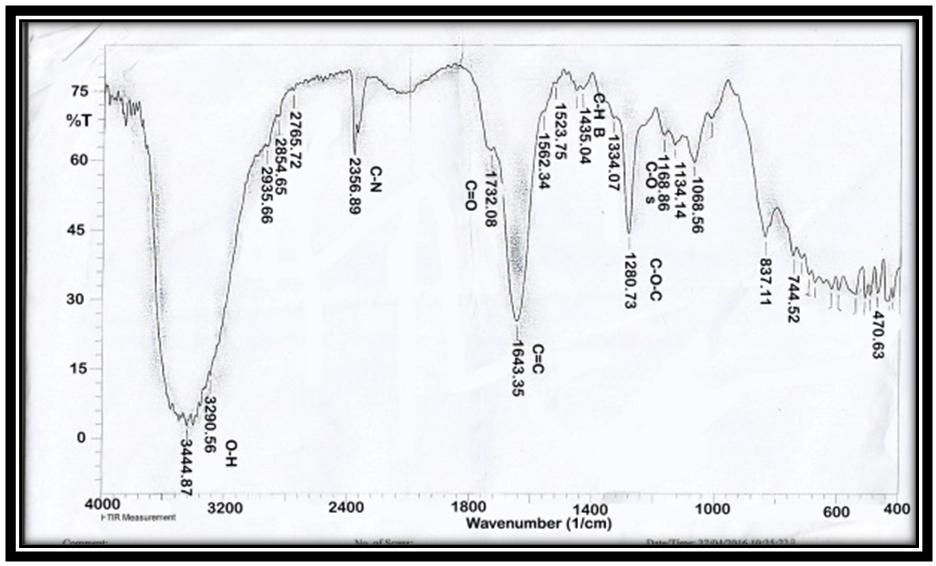 | Figure 3. FT-IR of AAV at ratio [1:1] |
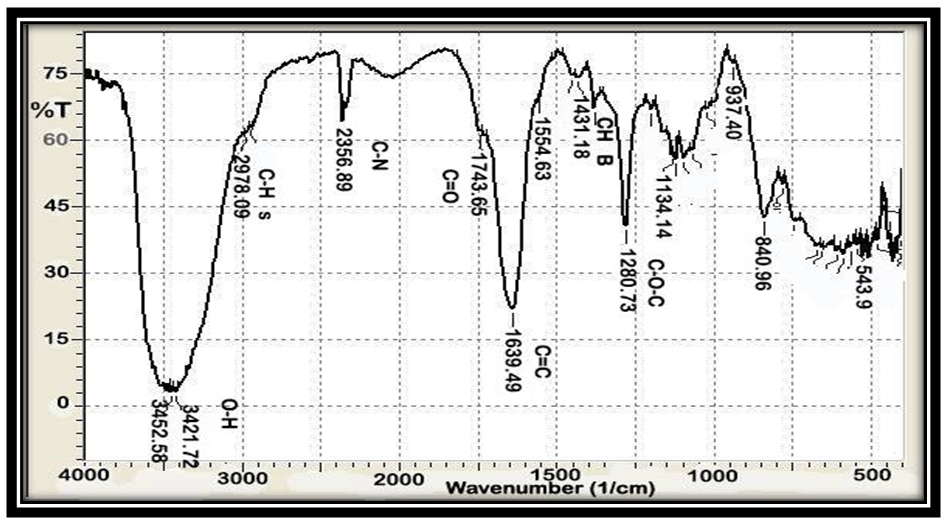 | Figure 4. FT-IR of AAV at [1:2] |
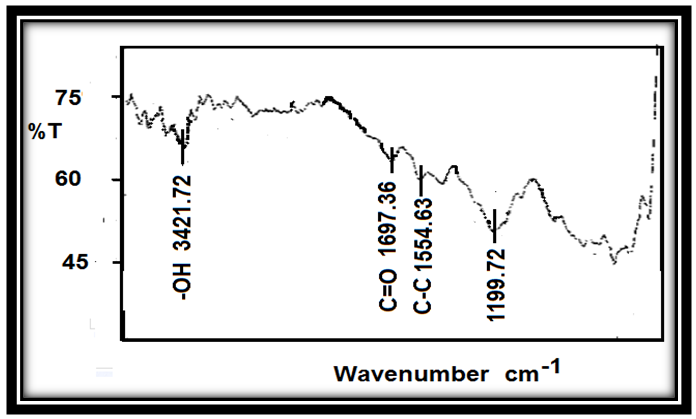 | Figure 5. FT-IR of MWCNTs |
|
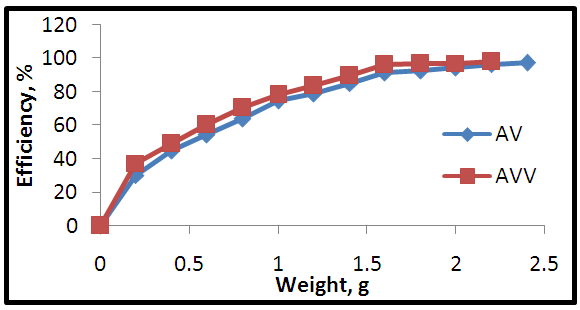 | Figure 6. Effect of different adsorbent dosage on the adsorption process |
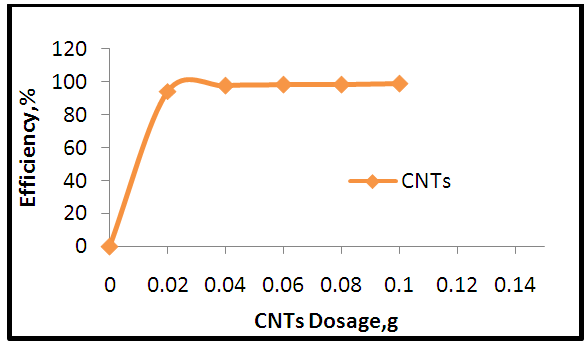 | Figure 7. Effect of MWCNT dosage on the adsorption process |
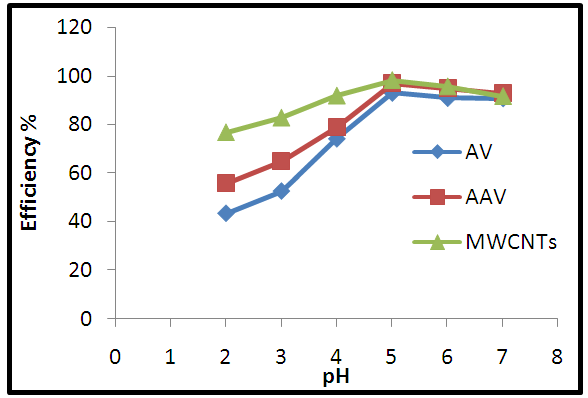 | Figure 8. Effect of pH on adsorption process |
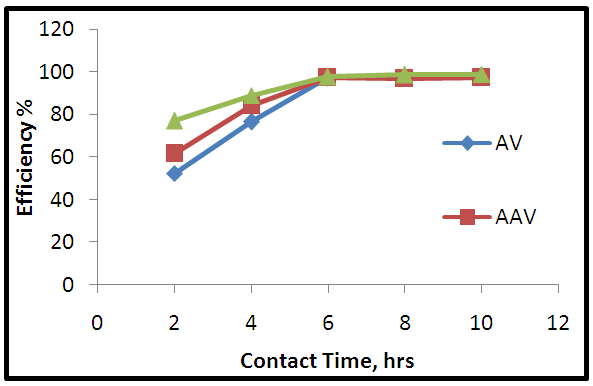 | Figure 9. Effect of contact time in adsorption process |
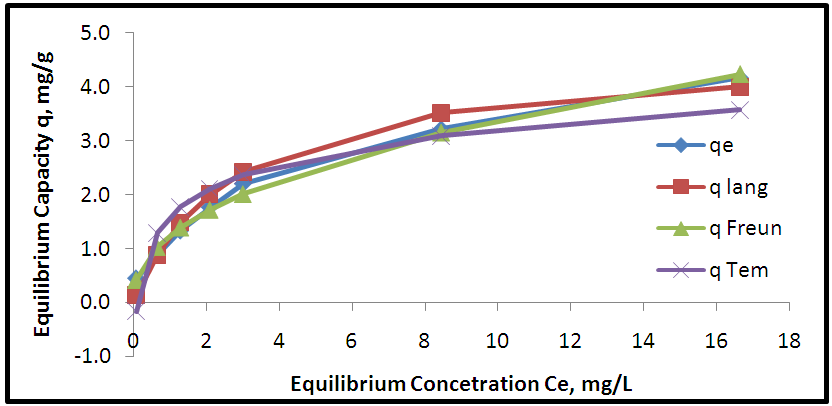 | Figure 10. Adsorption isotherms of experimental and theoretical models for adsorption of Pb onto AV |
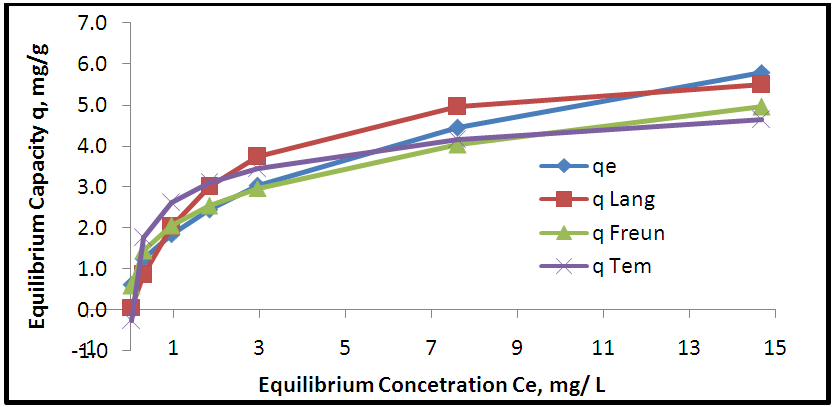 | Figure 11. Adsorption isotherms of experimental and theoretical models for removal Pb onto AAV |
|
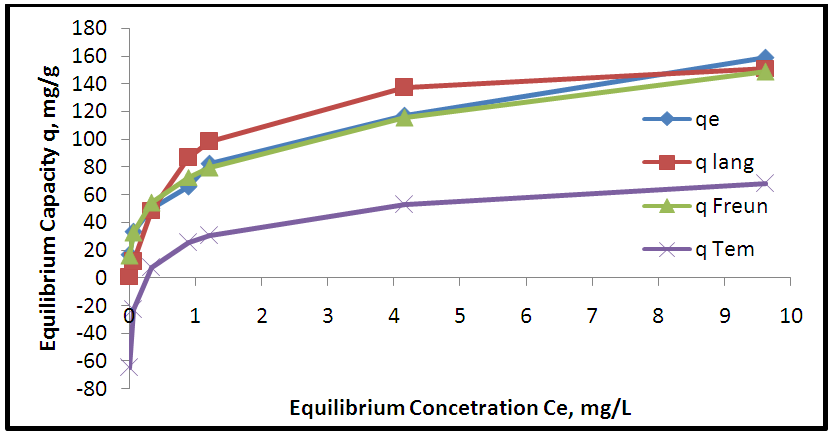 | Figure 12. Adsorption isotherms of experimental and theoretical models for the removal of Pb onto MWCNTs |
4. Conclusions
- Aloe Vera is a cheap, available and effective adsorbents for removal Pb+2 from aqueous solution. The maximum adsorption efficiency of removing lead ions is (96.49%) at 2.2 g of Aloe Vera. Acid activation Aloe Vera with acid [HNO3: H2SO4]. After activation of Aloe Vera leaves powder observe improvement in surface area from 13.8 m2/g to 24.6 m2/g and gave better removal efficiency of lead ions (96.27%) at 1.6 g. The functionaliztion of MWCNTS is an effective adsorbent for removing Pb+2 ion from aqueous solution. MWCNTs have large surface area (63.17 m2/g). The maximum adsorption efficiency (98.07%) at 0.06 g of MWCNTs. The maximum adsorption occurred at pH5 for lead. The maximum adsorption efficiency occurs after 6 hours for all adsorbent. The equilibrium data characterized by the Langmuir, Freundlich and Temkin isotherm. Isotherm equations and correlation coefficient R2 showed that Freundlich isotherm fits the experimental data better than Langmuir and Temkin for lead ions when using AV, AAV, MWCNTs as an adsorbent.
ACKNOWLEDGEMENTS
- The authors would like to acknowledge the Engineering Technical College-Baghdad for their encouragement, help and their interest in my project.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML