-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2016; 6(4): 105-114
doi:10.5923/j.materials.20160604.04

Removal of Zinc Ions from Aqueous Solution by Bioadsorbents and CNTs
Ahmed A. Moosa, Ali Mousa Ridha, Noor Amir Hussien
Department of Materials Engineering Technology, Engineering Technical College– Baghdad, Middle Technical, University Baghdad, Iraq
Correspondence to: Noor Amir Hussien, Department of Materials Engineering Technology, Engineering Technical College– Baghdad, Middle Technical, University Baghdad, Iraq.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In the present work is concerned with the use of Aloe Vera leaves powder (AV), activated Aloe Vera powder (AAV) and multi walled Carbon nanotube (MWCNTs) as an adsorbents for the removal of Zn2+ from aqueous solutions in batch adsorption system. The characterization of Aloe Vera (AV), activated Aloe Vera leaves powder (AAV) and multi walled Carbon nanotube (MWCNTs) was performed by Brunauer, Emmet and Teller (BET) for surface area, and Fourier-transformation infrared spectra (FTIR) for functional groups. The effect of different parameters such as adsorbent dosage, pH of adsorbate solution and contact time were studied for removal of Zn2+ from aqueous solutions onto each of adsorbent of AV, AAV and MWCNTs. The results show that the adsorption efficiency of Zn2+ removal onto each of AV, AAV and MWCNTs increases with the increase of the adsorbent dosage, pH of adsorbate solution and contact time. The best conditions for Zn onto each of adsorbents and the maximum adsorption efficiency were obtained to be 58.54% for AV at (2.2 g, pH 5 and 6 hr), 80.83% for AAV at (1.6 g, pH 5 and 6 hr) and 96.27% for MWCNTs at (0.06 g, pH 5 and 6 hr).The equilibrium isotherm of all adsorbents shows that the Freundlich isotherm gives a better fit to the experimental data than Langmuir and Temkin adsorption isotherm.
Keywords: Adsorption, Heavy metals, Zn 2+ removal, Bioadsorption, Carbon Nanotube
Cite this paper: Ahmed A. Moosa, Ali Mousa Ridha, Noor Amir Hussien, Removal of Zinc Ions from Aqueous Solution by Bioadsorbents and CNTs, American Journal of Materials Science, Vol. 6 No. 4, 2016, pp. 105-114. doi: 10.5923/j.materials.20160604.04.
Article Outline
1. Introduction
- Water pollution is a dangerous environmental problem. It’s one of major problem that concern the world at all. Water pollution occurs when water source are discharged by pollutants in directly or indirectly way (Owa and F.D 2013, Mann et al. 2014). With the high industries development, be clearly the heavy metals wastewaters are discharging into environment was increasing. Unlike organic contaminants, heavy metals are not biodegradable and tend to be toxic or carcinogenic to the living organisms (Fu and Wang 2011).The source of heavy metals in the environment are come from several industrials activities involves mining, smelting, surface finishing industry, metallurgy etc. The heavy metals are toxic at low concentration and directly related with biological toxicity problems and environmental pollution (Jemba 2004). Pollution could be reducing by employed with these practices (i) Recycling (ii) Reusing (iii) Waste minimization (iv) Mitigating (v) Preventing (vi) and Compost (Owa and F.D 2013, Mann et al. 2014). Zinc is one of most hazardous metal that is being widely used. Zinc is released into the environment from various industrial activities such as galvanizing plants, ores and municipal wastewater treatments (Kumar 2013). World Health Organization standards that identified the maximum concentration of the Zinc in the drinking water are 5.0 mg L−1 (WHO, 2003).The treatment of heavy metals in wastewater and drinking water is utilized to reduce the heavy metal levels to the acceptable concentration. Several conventional methods are available with a varying degree of success to remove heavy metals ions from wastewater. Some of them are chemical precipitation (Esalah et al. 2000), ion exchange (Lin et al. 2008), filtration (Bessbousse et al. 2008), solvent extraction (Gupta et al. 2007), reverse osmosis (Benito et al. 2002), and adsorption (Aguado et al. 2009). However, most of these methods are either ineffective, especially at low concentrations of heavy metals, or duo to it expensive. In addition, the sludge generated by these methods are toxic and the waste products required proper disposal and further treatment (Kumar 2013, Nuhoglu & Malkoc 2009). The increase of heavy metal pollution in wastewater lead to use an economical method like adsorption process (Fu and Wang 2011). Adsorption process has offered flexibility in operation and design, while in many cases will produce high-quality treated effluent. In addition, adsorption is sometimes reversible process, adsorbents can be reformed by the suitable desorption process therefore adsorption mostly method applied to remove metals (Fu and Wang 2011).The use of agriculture waste for removal heavy metals is called a bio-sorption (Barakat 2011). Due to use agricultural materials of bio-sorption and cellulose is responsible for the process of adsorption (Kumar et al. 2013).Aloe Vera (AV leaf powder) is a plant, which belongs to the family of Liliaceae and is generally succulent with a roll of elongated. This plant has very long age, which found a lot in India (Malik et al. 2015). Aloe leaf consists above 75 nutrients and 200 active compounds, including 20 minerals, 18 amino acids, and 12 vitamins like A, B1, B2, B12, E and C. (Mulu et al. 2015).The application of nanomaterials of environmental pollution for removing of toxicity materials due to the High surface area and surface reactivity of nanomaterials (Bhattacharya et al. 2013). In addition use carbon nanotube in adsorption process because of special morphology, chemical purification, role player of defect (Serp et al. 2003). The aim of this work is to study the removal of Zn+2 from wastewater using different adsorbents: Aloe Vera (AV), activated Aloe Vera (AAV) and multi walled Carbon nanotube (MWCNTs) and compare their adsorption efficiency. The influence of adsorbent dosage, pH and contact time on the adsorption efficiency of Zn+2 were investigated to obtain the better operating conditions of adsorption process. The adsorption isotherm models namely Langmuir, Freundlich and Temkin were studied to describe the adsorption equilibrium.
2. Material and Methods
2.1. Adsorbate
- Zinc Nitrate Hexahydrate salt Zn (NO3)2.6H2O was used to prepare 1000 mg/L standard stock solution of Zn (II) ion solution. 4.54 g zinc nitrate hexahdrate was dissolved into distill water with stirring to obtain 1000 ml of of zinc ions solution. The atomic absorption spectrometer showed that the concentration of zinc ions solution is 1000 ppm. The pH of the zinc ions initial concentration was adjusted to a certain pH by adding NaOH or HCl.
2.2. Adsorbents
2.2.1. Aloe Vera Plant
- Fresh green Aloe Vera leaves were collected from local garden. The collected leaves were washed extensively with distiller water for several times to remove dust. These leaves were cut into small pieces (1 x 1 cm) and allowed to dry in the sun for 5 days in order to remove water and the preservation of the Aloe Vera components. Then the leaves were dried in an oven at 50°C for 2 hours. The dried Aloe Vera leaves were then ground using a home electrical grinder into fine powder.
2.2.1.1. Activation of Aloe Vera Plant
- Aloe Vera powder was acid activation by using [HNO3: H2SO4] at different ratio [2:1], [1:2] and [1:1]. About 1 g of Aloe Vera powder was added to 20 ml of acid solution and then mixed using magnetic stirrer for 6 hrs at 120 rpm. After that Aloe Vera acid solution was left for 24 hrs at room temperature followed by washing several times with distal water until pH reached a constant value. After this treatment, the modified Aloe Vera was dried in a furnace at 80°C for 6 hrs and the obtained acid activation Aloe Vera (AAV).
2.2.2. Multi Walled Carbon Nanotube (MWCNTs)
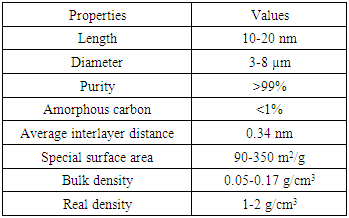
- High Purity 99%, Multi Wall Carbon Nanotubes (MWCNTs), Intelligent Materials Pvt. Ltd., Punjab, India will be used as adsorbent. The specifications of MWCNTs are shown in Table 1.
|
2.2.2.1. Functionalization of MWCNTs
- 0.5 g of MWCNTs were immersed in 500 ml of mixture of concentrated (HNO3:H2SO4) (1:3 by volume) at room temperature. The MWCNTs solution with acid was ultrasonicated using ultrasonic path at frequency 60 KHz, power 140 W) for 40 min at 40°C. The solution was then diluted 15 times with deionizer water and then filtered using vacuum filtration system with 0.2 μm pore size filter papers. This washing operation was repeated until the pH became the same as that of deionized water. The MWCNTs on the filter paper was then left to dry at room temperature for 12 hours. After drying a sharp blade is used to remove CNTs from filter paper and then dried in an oven at 60°C for 6 hrs (Moosa et al. 2010) (Moosa et al. 2015).
2.2.3. Characterization of Adsorbents
- The specific surface area of AV, AAV, and MWCNTs were measured using the BET analyzers (model 9600, USA). AV, AAV and MWCNTs were characterized using the Fourier-Transformation Infrared Spectra (FT-IR) (IRTracer-100 / Shimadzu Co. / USA).
2.2.4. Adsorbent Dosage
- To determine the optimum weight of different adsorbents dosage for adsorption of Zn (II) onto Aloe Vera, acid treated Aloe Vera powder and MWCNTs were obtained experimentally. Different dosage (0.2, 0.4, 0.6, 0.8, 1,1.2, 1.4, 1.6, 1.8, 2, 2.2, 2.4g) for Aloe Vera and activated Aloe Vera powder, and for MWCNTS (0.02, 0.04, 0.06, 0.08, 0.1) g was added to 50 ml with initial concentration of 100mg/L of Zn2+. The initial pH of Zn2+ solutions was fixed at 4.5. The solution was placed in the bottles and then agitated for about 5 hrs in a shaker with water bath at agitation speed of 125 rpm and the temperature was maintained at 25°C. The samples were then filtered and the remaining concentration of Zn2+ was measured by atomic-absorption spectrophotometer. The best dosage of AV, AAV and MWCNTs was chosen at maximum adsorption efficiency (maximum removal).
2.2.5. Effect of pH
- To obtain the optimum pH of Zn (II) solution adsorption onto AV, AAV and MWCNTs a series of batch experiments were conducted at various pH in the range of (2, 3, 4, 5, 6, and 7) for Zn(II). The best dosage of AV, AAV and MWCNTs was added to 50 ml with initial concentration of 100mg/L of Zn2+. The pH of solutions was adjusted to desired value using dilute of 1M NaOH or 1M HCl. The solution was placed in the bottles and then agitated for about 5 hrs in a shaker with water bath at agitation speed of 125 rpm and the temperature was maintained at 25°C. The samples were then filtered and the remaining concentration of Zn2+ was measured by atomic-absorption spectrophotometer. The best pH of zinc solution was then obtained at maximum adsorption efficiency (maximum removal).
2.2.6. Effect of Contact Time
- The effect of contact time on the adsorption of Zn(II) onto Aloe Vera, acid activated Aloe Vera powder, and MWCNTs was studied using various adsorption times (2, 4, 6, 8 and 10 hr). The solutions of Zn was adjusted to the best pH with the initial concentration 100 mg/L. A volume 50 mL of Zn (II) solutions were placed in the bottles. The best dosage of each adsorbents were added to each bottle. The solution in the bottles was agitated for different times in a shaker with water bath at agitation speed of 125 rpm and at 25°C and then filtered. The equilibrium concentration of zinc ions were determined by atomic-absorption spectrophotometer. The best time was then obtained at maximum adsorption efficiency (maximum removal).
2.2.7. Equilibrium Isotherm
- The equilibrium isotherms for adsorption of Zn+2 ions onto Aloe Vera, acid activated Aloe Vera and MWCNTs were performed experimentally using the best dosage for each adsorbents, the best pH of Zn+2 ions solutions and best adsorption period. The solutions of Zn+2 ions with different initial concentration (20, 40, 60, 80, 100, 150 and 200 mg/L) were adjusted to the best pH of Zn+2 ions solutions. A 50 ml of Zn+2 ions solutions were placed in the bottles. Best dosage of Aloe Vera, acid activated Aloe Vera and MWCNTs were added to each bottle. The samples in bottles were agitated at best adsorption period in a shaker with water bath at agitation speed 125 rpm and the temperature was maintained at 25°C. The samples were then filtered and the remaining concentration of zinc ions were measured by atomic-absorption spectrophotometer.
2.2.7.1. Langmuir Isotherm Model
- Langmuir model considered the first theoretical of nonlinear sorption, which sorption occur on a homogenous surface without interaction between adsorbed molecules (Dada et al. 2012).The Langmuir isotherm equation is (Dada et al. 2012)
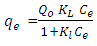 | (1) |
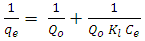 | (2) |
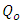 is a maximum capacity of active site (mg/g)KL is the Langmuir constant (L/mg).
is a maximum capacity of active site (mg/g)KL is the Langmuir constant (L/mg).2.2.7.2. Freundlich Isothermal
- The Freundich (1906) is an equation depended on adsorption on a heterogenous surface, this model is applied to calculate the adsorption intensity of the adsorbate on the adsorbent (Kalalagh et al. 2011).The Freundlich model equation is (Kalalagh et al. 2011)
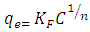 | (3) |
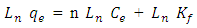 | (4) |
2.2.7.3. Temkin Isotherm
- The Temkin (Temkin, 1940) isotherm equation supposes that the heat of adsorption of all the molecules in layer reduces linearly with covering due to adsorbent-adsorbate interactions, and that the adsorption is described by a uniform distribution of the bonding energies, until some maximum binding energy (Kalalagh 2011). Temkin model represented by (Kalalagh 2011).
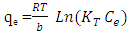 | (5) |
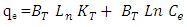 | (6) |
3. Results and Discussion
3.1. FT-IR
- The functional groups present in Aloe Vera leaves were carried out in the range of 400 to 4,000 cm−1 is shown in Figure 1. The presence of strong and broad intensity band around (3410.15 cm-1) was assigned to phenolic OH stretching (Ray and Gupta 2013) and (Ray et al. 2013). The peak at 2924.09 cm-1corresponds to -CH stretching that might be due to H-bond formation at higher concentrations. The absorbance bands were seen in the range of 1732.08 cm-1 and 1269.16 cm−1 which attributed to C=O and C-O-C stretching of acetyl groups present in the sample (Kiran and Rao 2014). Moreover, the bands at 1650–1578 cm−1 implied the presence of C=O stretching and indicated the presence of carboxyl components and phenolic compounds which are polar in nature (Ray and Gupta. 2013) and (Ray et al. 2013). The absorbance spectrum at around 1732.08 cm−1, 1597.06 cm−1, and 1269 cm−1 can be accounted for the presence of C=O, COO-, and C-O-C stretches of acetyl groups (Hulle et al. 2014) and (Femenia et al. 2003). The CH3 and COO (corresponds to 1431.18 cm−1). The identification of strong bands in the range of 1076.26-1033.85 cm-1 due to the presence of polysaccharide sugars, such as galactose and glucans (Barandozi & Enferadi 2012).
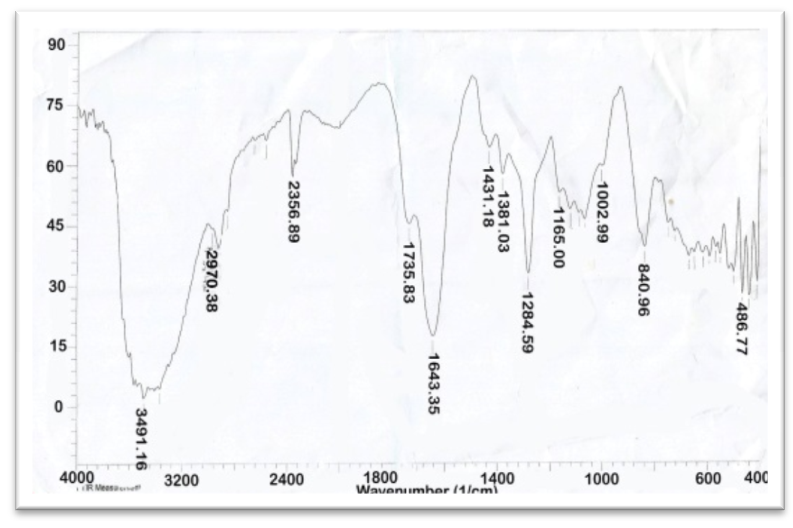 | Figure 1. FT-IR of Aloe Vera |
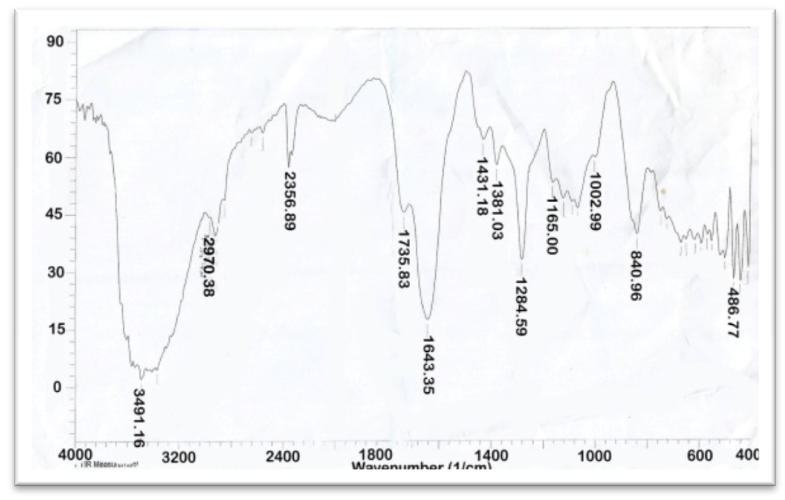 | Figure 2. FT-IR of AAV at [2:1] |
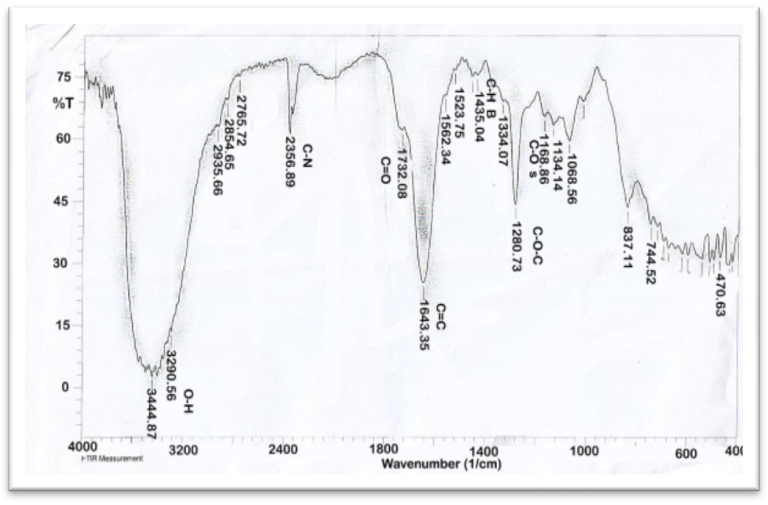 | Figure 3. FT-IR of AAV at ratio [1:1] |
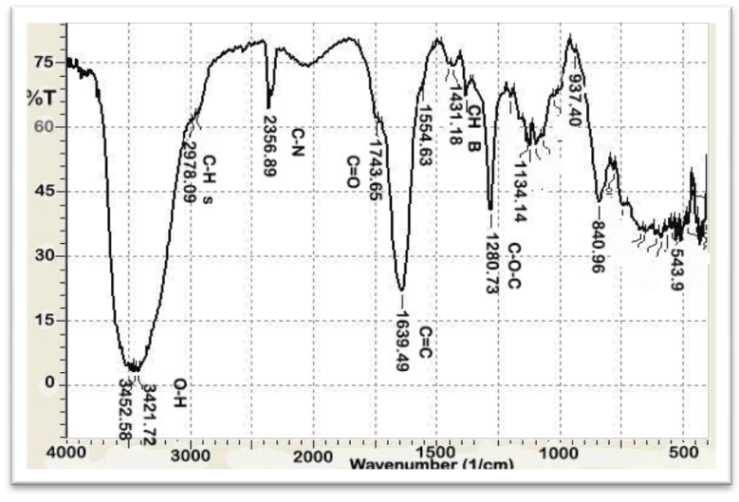 | Figure 4. FT-IR of AAV at [1:2] |
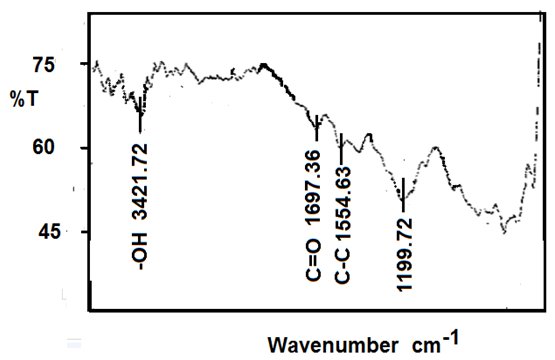 | Figure 5. FT-IR of MWCNTs |
3.2. Surface Area of Biosorbents and MWCNTs
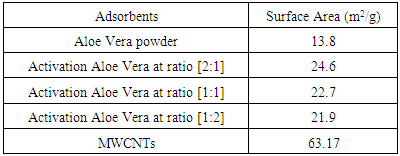
- Aloe Vera leaves powder was acid activated with mixture [HNO3:H2SO4] at different ratio [1:2], [2:1] and [1:1] at 25°C. The surface area of acid activation of the Aloe Vera leaves powder using BET analysis is shown in Table 2. The results show that the acid activation of the Aloe Vera caused some modifications in the structure of the Aloe Vera may be increase irregular surface and porous structure. The surface area of Aloe Vera powder is 13.8 m2/g. The result offers that the surface area increased as the concetration of the acid treatment increased thereafter decreased with further increase in the concentration of acid activation (Ajemba 2013). At ratio [2:1] observed large surface area (24.6 m2/g) compare with other activation ratio.
|
3.3. Effect of Adsorbents Dosage
- The effect of different dosages (0.2, 0.4, 0.6, 0.8, 1, 1.2, 1.4, 1.6, 1.8, 2.2, 2.4 g) of each AV and AAV, (0.02, 0.04, 0.06, 0.08, 0.1)g of MWCNTs are show in Figures 6 and 7 respectively. From this Figure 6, it can be seen that the adsorption efficiency was increased gradually when the AV dosage increase, which varied from 14.57% to 59.36% ions. The adsorption efficiency of Zn increased gradually up to 2.2 g of AV. A further increase in AV dosage beyond 2.2g shows no enhancement in adsorption efficiency of Zn due to reach the equilibrium state between the solid (adsorbente) and liquid (adsorbate). This behavior can be explained by available number of adsorption sites and the surface area increases with increasing the adsorbent weight. The above results are confirmed by (Abudaia et al. 2013). The best dosage of AV was obtained at 2.2 g for removing zinc ions.
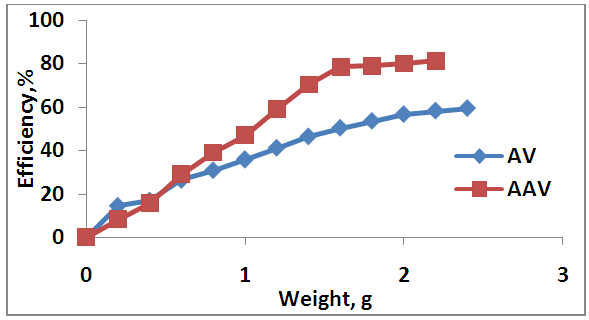 | Figure 6. Effect of different adsorbents dosage on adsorption process |
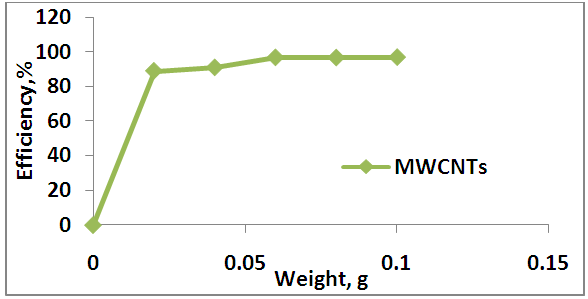 | Figure 7. Effect of MWCNTs dosage on adsorption process |
3.4. Effect of pH
- The effect of different pH of Zn solution (2, 3, 4, 5, 6, 7) onto AV, AAV and MWCNTs are show in Figure 8.
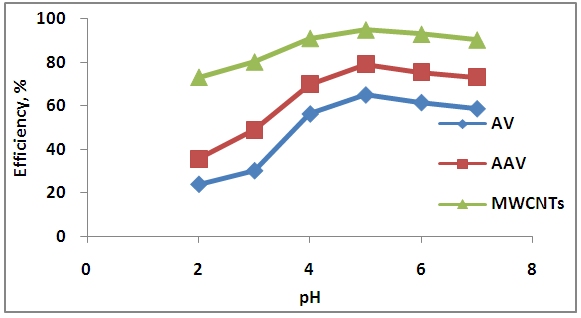 | Figure 8. Effect of pH on adsorption process |
3.5. Effect of Contact Time
- The effect of different contact time (2, 4, 6, 8 and 10) hr onto adsorption process by using AV, AAV and MWCNTs are shows in Figure 9.
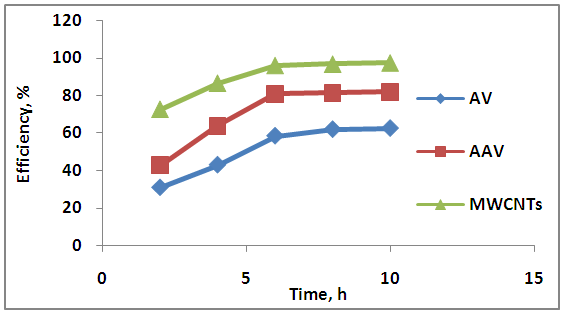 | Figure 9. Effect of contact time on adsorption process |
3.6. Equilibrium Isotherm
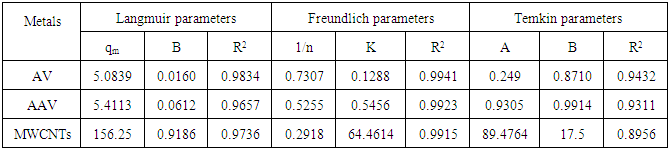
- The experimental results of the adsorption isotherm curves were obtained by plotting capacity of adsorption against the equilibrium concentration of the adsorbate. Different initial concentration of Zn ions (20, 40, 60, 80, 100, 150, 200 mg/L) were used at pH 5, and 6 hr contact time. Figures 10 show the adsorption isotherm curves for Zn+2 ions onto AV at 25°C respectively. The experimental data for Zn+2 adsorption onto A V are correlated with the three theoretical adsorption isotherm models. These figures characterize the experimental data and the theoretical data obtained from Langmiur, Freundlich and Temkin isotherm. All constants and correlation coefficients for Langmiur, Freundlich and Temkin isotherm theoretical models are listed in Table 3.
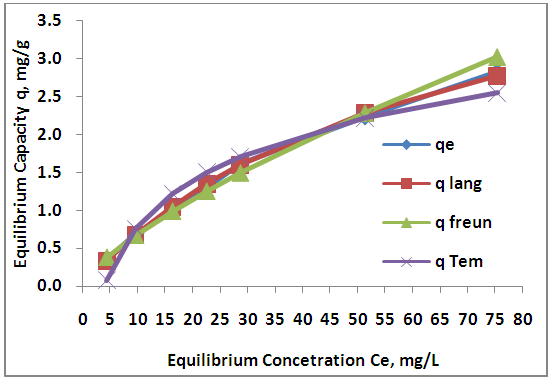 | Figure 10. Adsorption isotherms for Zn (II) by Aloe Vera |
|
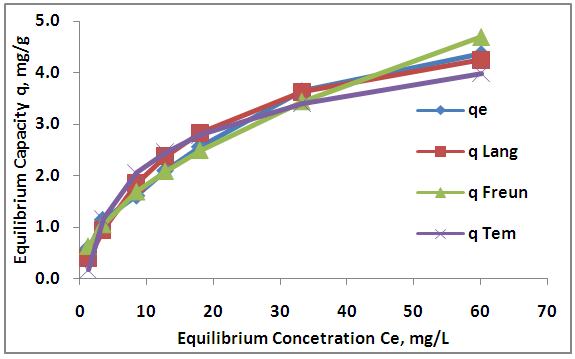 | Figure 11. Adsorption isotherms for Zn (II) by activation of Aloe Vera |
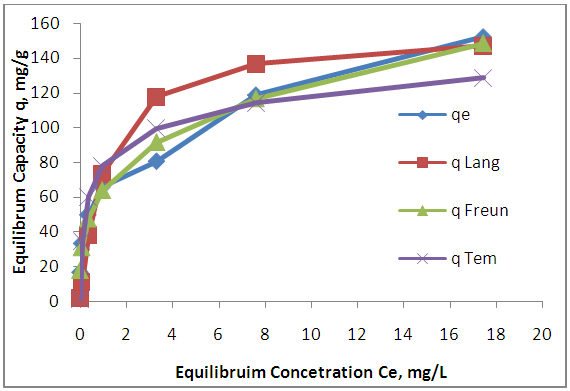 | Figure 12. Adsorption isotherms for Zn (II) by MWCNTs |
4. Conclusions
- MWCNTs showed higher adsorption efficiency than AV and AAV for the removal of Zn+2 from aqueous solution due to large surface area. The maximum adsorption efficiencies for zinc are (53.49%, 78.5% and 96.65%) at dosage (2.2, 1.6, 0.06) g of AV, AAV and MWCNTs respectively. The maximum adsorption occurred at pH 5 after 6 hours. The equilibrium data of isotherm showed that Freundlich isotherm fits the experimental data better than Langmuir and Temkin for zinc ions when using AV, AAV, MWCNTs as adsorbents.
ACKNOWLEDGEMENTS
- I begin to thank God for your patience and conciliation to me during my studies. Authors would like to acknowledge Engineering Technical College-Baghdad for their encouragement, help and their interest in my project.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML