-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2016; 6(4): 87-90
doi:10.5923/j.materials.20160604.01

Properties and Characterizations of Different Natural Jordanian Materials
Murad Alsawalha
Department of Chemical & Process Engineering Technology, Jubail Industrial College, Jubail Industrial City, KSA
Correspondence to: Murad Alsawalha, Department of Chemical & Process Engineering Technology, Jubail Industrial College, Jubail Industrial City, KSA.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

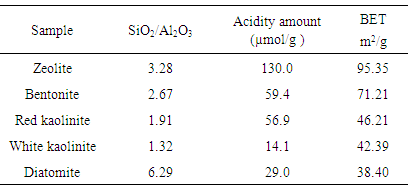
This paper focused on characterizations of different natural Jordanian clays containing zeolite, bentonite, red white kaolinite and, diatomite by different instruments like temperature programmed desorption (TPD) of ammonia, FTIR and, surface area. It has been found that the decrease of surface acidity correlates with the decrease of Si/Al ratio with exception of diatomite and the surface area order was found as follows; Zeolite, Bentonite, red kaolinite, white kaolinite and Diatomite.
Keywords: Clay’s characterizations, FTIR, Surface area, Instrumental characterization
Cite this paper: Murad Alsawalha, Properties and Characterizations of Different Natural Jordanian Materials, American Journal of Materials Science, Vol. 6 No. 4, 2016, pp. 87-90. doi: 10.5923/j.materials.20160604.01.
Article Outline
1. Introduction
- Characterization of acidic properties of inorganic solids is essential for understanding of the behavior of catalysts in heterogeneously catalyzed reactions [1, 2]. The acidity of alumosilicates is characterized in terms of Broensted and Lewis acid sites. Brønsted acid sites are formed by aluminum atoms connected to silicon by a so-called ‘‘bridging hydroxyl’’ Al–(OH)–Si where the negative charge generated is compensated for by a proton. Lewis acid sites are composed of aluminum with low coordination or ≡Si+ ions formed from dehydroxylation in the thermal treatment at T > 773 K [3]. Therefore, the acidity of an alumosilicate is related to its silica and aluminum contents, and increases linearly with increasing silica to aluminum ratio in the sample. Acidic and basic properties of porous materials such as zeolites, clays or mesoporous silica are usually determined by titration and spectroscopic techniques (FTIR, XPS, NMR) [4-6], together with temperature programmed desorption (TPD) of probe molecules such as NH3 and CO2 [7-9]. For the last decades catalytic test reactions have been intensively used as a tool for characterization of acid-basic properties of solids [10], [11] under real as practice-near as possible conditions. The first model reactions such as n-hexane conversion or but-1-ene isomerization were proposed to characterize acidity of solids to be used for petroleum chemistry [12]. Another powerful test reaction for clarification of structural questions like the conversion of 2-methyl-3-butin-2-ol (MBOH) was suggested and tested by [14]. According the acid-base properties of catalysts MBOH is converted to different products for each reaction pathway, namely 3-methyl-3-buten-1-yne (MBYNE) or 3-methyl-2-buten-1-al (Prenal) at acid sites and acetone together with acetylene at basic sites. Investigations on the MBOH conversion were performed on solids like zeolites hydrotalcites, magnesium oxide catalysts, and others like clays [13-16]. Natural clay minerals are well known and familiar to humankind from the earliest days of civilization. Because of their low cost, abundance in most continents of the world, high sorption properties and potential for ion exchange, clay materials are strong candidates as adsorbents. Clay materials possess a layered structure and are considered as host materials. They are classified by the differences in their layered structures and properties.In this study, different instruments were utilized to describe relevant features properties of raw Jordanian clays; temperature program description of ammonia, FTIR and, BET surface area.
2. Experimental
2.1. Materials
- Chemical composition of these samples was determined by X-ray Fluorescence (XRF) method [2]. It was performed to know the chemical compositions of the minerals that are present in the different five samples of Jordanian clays containing zeolite, bentonite, white kaolinite, red kaolinite and diatomite were collected from National Resource Authority, Jordan [17-19].
2.2. Characterization Methods
2.2.1. Temperature Programmed Desorption of Ammonia (TPD- NH3)
- Raw Jordanian clays samples were characterized by NH3-TPD with an apparatus constructed from Raczek analyze technical GmbH, Hannover. The apparatus consists of an adjustable oven to heat the sample, gas supplies for helium and a mixture of 5 vol.% NH3 in argon (Messer-Griesheim) and a thermal conductivity detector (TCD) for gas analysis [15]. Prior to adsorption of ammonia 0.6 g of each samples was heated within a U-tube under He stream at a rate of 20°C/min from room temperature up to 600°C. Subsequently the sample was cooled down to 40°C and saturated in flow of gas mixture containing 8 vol.% NH3/Ar for 30 min. The physically adsorbed ammonia was desorbed from the sample in a stream of He at 100°C for 32 min. Desorption of the chemically adsorbed part of ammonia was realized by increasing temperature from 120°C up to 460°C at a rate of 25°C/min.
2.2.2. BET Measurements
- The surface area of samples were characterized by the BET method, performing adsorption of nitrogen at – 196°C with the apparatus Quantachrome Autosorb-1. The Quantachrome autosorb-1 operates by measuring the quantity of nitrogen adsorbed onto the solid surface at some equilibrium vapour pressure by the static volumetric method. The data are obtained by admitting a known quantity of adsorbate gas, which is nitrogen, into the sample cell containing the solid adsorbent maintained at a constant temperature below the critical temperature of the adsorbate. As adsorption occurs the pressure on the sample cell changes until equilibrium is established. The quantity of gas adsorbed at the equilibrium pressure is the difference between the amount of gas admitted and the amount required to fill the surface of the adsorbent. Data acquisition, reduction and calculating were performed by Quantachrome Autosorb software.
2.2.3. FTIR Measurements
- Analytical spectra were taken using a Shimadzu IR Prestige-21/FTIR-8400S spectrophotometer through the wave number range from 400 to 4000 cm-1 using the KBr pellet technique. The spectra were collected using Shimadzu IR Prestige-21 Windows Software.
3. Results and Discussions
3.1. FTIR-study
- FTIR studies of these natural materials help in the identification of various forms of the minerals present in the clay.Experimental FTIR-spectra of Jordanian clays can be characterized by three main adsorption regions, i.e. adsorption bands within the hydroxyl region 3400-3740 cm-1, OH-bending of physically adsorbed water at 1634 cm-1 and low frequency range bands 400-1100 cm-1 caused by structural alumosilicate frameworks. Sharp bands at 3690 and 3614 cm-1 are assigned to OH-vibrations of free Si-OH groups of diatomite and kaolinite [18], [20]. A band at 3620 cm-1 on the zeolite spectra indicates on the acidic bridging hydroxyl Si-O(H)-Al. Wide bands at 3430 cm-1 at all spectra orrespond to OH-vibration of physically adsorbed water. A band at 1634 cm-1 corresponds to deformation vibration of adsorbed water [21]. Vibration modes of siloxane (Si-O-Si) stretching are found to be around 1088 and 1026 cm-1. The bands of Si-O stretching are discerned for diatomite at 910 and 800 cm-1. For zeolite, bentonite and kaolinite the bands in the low frequency region are hard to discern, while for diatomite the peaks at 466 cm-1 and 524 cm-1 corresponded to a Si-O-Si and Al-O-Si deformation respectively [18], [20]. It is worth mentioning that due to the analogical vibration frequencies of different surface hydroxyl groups and overlapping by the broad peak of the adsorbed water (with middle wave number at ca. 3500 cm-1) it is difficult to distinguish the IR-spectra of alumosilicates [22]. The relation between free and H-bonded hydroxyls and adsorbed water molecules is changed under different thermal treatment conditions and affect the number of acid sites on the alumosilicate surface [23].
3.2. Temperature Programmed Desorption of NH3
- Experimental TPD-NH3 profiles of raw Jordanian clays are shown in figure 1. It can be seen from fig. 1 that desorption of ammonia from the samples starts at 100°C and reaches its maximum at temperatures of about 180°C for zeolite and bentonite and about 250°C for white and red kaolinite.
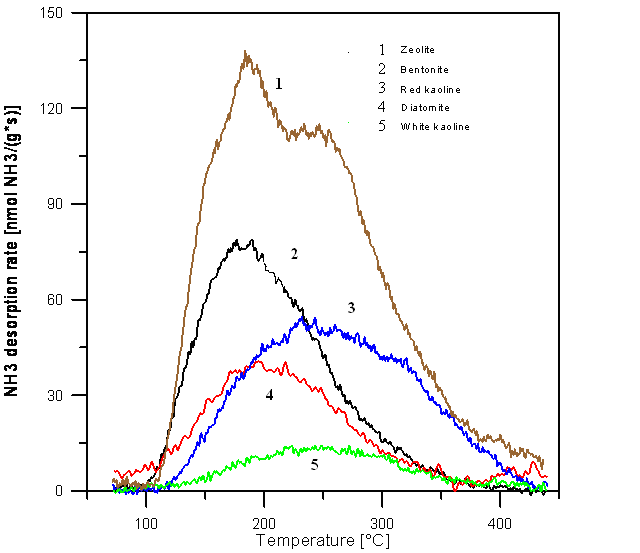 | Figure 1. Profiles of temperature programmed desorption of ammonia for different Jordanian clay samples |
|
4. Conclusions
- The current work has shown properties of various natural Jordanian clays by different techniques to illustrate several important aspects in the basic chemistry, such as material sciences, characterization techniques, adsorption processes (adsorption isotherms, surface area) and, identification of various forms of the minerals present in the clay.It was also concluded that Zeolite sample demonstrated maximum of ammonia desorption rate while, the lowest ammonia desorption was obtained from white kaolinite.
ACKNOWLEDGEMENTS
- Dr. Murad Alsawalha gratefully acknowledges Professor Frank Rößner at Carl Von Ossietzky (Germany), Institute for Pure and Applied Chemistry, Industrial Chemistry, for his valuable scientific recommendations. The author also acknowledges Carl Von Ossietzky University facilities.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML