-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2016; 6(3): 79-85
doi:10.5923/j.materials.20160603.03

Improvement Some Physical Properties of Egyptian Battery Grids for Industrial Applications: Comparison Study with German Battery Grids
1PhD Researcher at Mansoura University, Faculty of Science, Physics Department, Metal Physics Lab, Egypt
2Assistant Lecturer, University of Science and Technology, Sanaa, Yemen
Correspondence to: Mohammed S. Gumaan , PhD Researcher at Mansoura University, Faculty of Science, Physics Department, Metal Physics Lab, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The alloys of Pb-Sb-Sn with Ca and Al were produced by the melt-spinning technique and compared with the pure lead, Egyptian battery grid and German battery grid which are produced by the same method. X-ray diffraction analysis (XRD) was implemented. The mechanical and electrical properties were measured. It was found that, there is a great benefit to be used melt-spinning technique in numerous commercial melt-spun ribbon alloy systems, also it's found that the antimony rich precipitates in the structure and the microstructural changes caused by rapidly solidified strengthens the material, so Young’s modulus was found in Pb-Sb-Sn higher than Egyptian and German grid battery. The Ca and Al additions to this alloy was investigated and shown negative impacts, On the contrary the resistivity is lower for the Pb-Sb-Sn, so a rapidly solidified ternary Pb-Sb-Sn alloy is suitable alloy for the storage battery applications.
Keywords: Melt-Spun ribbons, German battery grid and Egyptian battery grid
Cite this paper: Mohammed S. Gumaan , Improvement Some Physical Properties of Egyptian Battery Grids for Industrial Applications: Comparison Study with German Battery Grids, American Journal of Materials Science, Vol. 6 No. 3, 2016, pp. 79-85. doi: 10.5923/j.materials.20160603.03.
Article Outline
1. Introduction
- The importance of Lead acid battery come from the primary functions of a storage battery in photovoltaic systems [1], it's recorded here as energy storing which produced by the photovoltaic and to supply energy to electrical loads on demand, supply power to electrical loads at stable voltage and currents and supply surge to electrical loads. The main component of these batteries is the metallic grid which is made up mainly of lead. Lead was one of the first metals known to man, it has many applications that are derived from being low tensile strength, high absorption of electromagnetic radiation, and it's highly resistant to corrosion, it's easy alloy ability. In everyday life, it has economically most important use, however comes from it favourable electrical properties, lead-acid storage batteries, the demise of which has been predicted for decades, are still being continuously improved [2-4]. The rationale for the usage of common alloying elements (Sb, Sn, Ca and Al) is determined empirically. Antimony is alloyed with lead in amounts typically ranging from 4 to 10 wt.% Sn in battery alloys with the Pb-Sb eutectic point at 11.1 wt.% which produce a microstructure consisting of an eutectic network within dendrites of primary Pb solid solution [5, 6]. The primary purpose of Sb additions is to produce strength in terms of solid solution strength in the Pb-rich phase, and via dispersion effect of a finely divided eutectic mixture. Tin is generally considered to add a favorable solid solution strengthening effect in Pb-Sb alloys, and perhaps to play some favorable electrochemical role [7], so the traditional alloys for grids in lead acid batteries have been used based on the lead – antimony alloys system. The use of low-antimony alloys has become important for low-maintenance batteries. These alloys, however, have lower castability and a reduced ability to age-hardening. A small addition of tin improves the fluidity and castability. It was reported that lead-calcium-tin alloys with low tin contents suffer from passivation. To reduce this phenomenon, plating with Pb-Sb-Sn alloy layer has been recommended [8]. The use of lead – calcium alloys has been investigated [9, 10], these alloys do not have the electrochemical problems of antimony bearing alloys, but have other drawbacks. They are somewhat weaker than antimonal alloys. They are difficult to cast, requiring high mould temperatures which slow production rates. Srivastava, UpadhYaYa and Ojha [11] have discussed the microstructural induced by spray forming a ternary Pb-Sn-Sb alloy. They are concluded that an alloy containing Pb-12%Sn-12%Sb with small addition of copper and arsenic was spray deposited employing two different atomization gas pressure and nozzle to substrate distance. Schouwenaars et al [12] reported that rapidly solidified industrially stripcast Pb-Sb-Sn showed microstructure for the eutectic composition of metallic glasses. Kamal et.al [13] reported that the Elastic modulus of Pb-Cu alloys increased with increasing Ca content whereas electrical resistivity values decreased with increasing Ca content. The fundamental reasons for the advantages and disadvantages of various alloy systems are evaluated and compared with that grids in lead acid industrial batteries fabricated in Egypt and in Germany. It is the first time to apply melt-spinning technique practically to the lead storage battery [14], because of this technique has the potential to control the particle size and shape and in many case drastically change the form and the nature of lead storage battery.
2. Material and Methods
- Rapid quenching from melt has become a major field of research activity in material processing. One main feature is to attain high cooling rates during the quenching process. The motivation of using high cooling rates lies in the resulting beneficial effects [15]; as the cooling rate in alloy solidification is increased, the microstructure of the product is more uniform, size-refined, extended solid solution, metallic glasses and non-equilibrium crystalline phase. The most widely used techniques for alloy production at high cooling rates are Chill methods [16], these techniques include chill-block melt-spinning technique which used in this investigation [17]. The experimental techniques utilized have been described in details in [18] and will be repeated here only briefly. Melt-spun ribbons thickness 80 μm to 120 μm, width about 4 mm, cooling rate about 105 K/sec [19-20], were prepared from Pb, Egyptian grid battery, German grid battery, Pb-10wt.%Sb-10wt.%Sn, Pb-9.5wt.%Sb-9.5wt.% Sn-1wt.%Ca and Pb-9wt.%Sb-9wt.%Sn-1wt.%Ca-1wt.%Al melt-spun alloys. A variety of technique was used to characterize the crystallographic, and transformation features of the melt-spun ribbons lead alloys including x-ray diffraction. The mechanical properties were examined in air atmosphere with a dynamic resonance method. The hardness of ribbons samples were measured on a Vickers micro- hardness tester (Modd FM7-Tech Group Tokoy-Japan). In-suite resistivity measurements have been carried out using the so-called double bridge method. The x-ray diffraction study was carried out using CuKα radiation at room temperature [21].
3. Results and Discussion
3.1. Structural Analysis
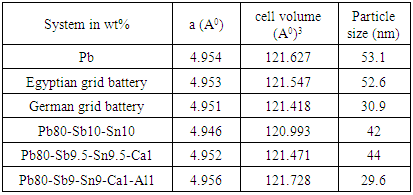
- The X-ray diffraction patterns for the as-quenched Pb, Egyptian grid battery, German grid battery, Pb-10wt.% Sb-10wt.%Sn, Pb-9.5wt.%Sb-9.5wt.%Sn-1wt.%Ca and Pb-9wt.%Sb-9wt.%Sn-1wt.%Ca-1wt.%Al, melt-spun alloys are shown in figure (1). For Pb, is shown in figure (1a) contains pure lead as quenched. For Egyptian battery lead storage as quenched ribbons figure (1b) contains pure lead-phase. For German battery lead storage as quenched ribbons figure (1c), It also contains the same phase pure Pb-phase. Figure (1d) reproduces an X-ray diffraction pattern of an quenched ribbons of Pb-10wt.%Sb-10wt.%Sn alloy. The pattern shows the existence of two kinds of phases with face centered cubic (fcc) structure of α-Pb solid solution and intermetallic compound from SbSn metastable phase. From these results, it has been achieved that the antimony tends to form intermetallic compound with the tin. For Pb-9.5wt.%Sb-9.5wt.%Sn-1wt.%Ca melt spun alloy is shown in figure (1e)contains fcc structure corresponding to α-Pb phase with a = 4.9466 A0. The X-ray diffraction patterns indicate a face- centered cubic Pb matrix phase, Sb-phase and SnSb intermetallic compound. Finally For Pb-9wt.%Sb-9wt.%Sn-1wt.%Ca-1wt.%Al melt-spun alloy is shown in figure (1f). The patterns show lines corresponding to α-Pb, Sb, and SbSn type phases. The intensity of Pb lines is indicating a significant concentration of this phase is the fully crystalline melt-spun ribbons. Increasing in start base line and curve broadening with Al addition are obtained. There is no any peak characteristic due to Ca-phase or Al-phase, which indicated that a complete solubility of Ca atoms or Al atoms in Pb-matrix phase has been well obtained, which lead to changing in lattice parameter value (a) and unit cell volume of Pb-phase in the Pb-Sb-Sn ternary alloy. This effect was interpreted as indication that cooling rate for the melt-spun ribbons used in this study as lead base alloys for high performance storage battery was high enough to retain most of the alloying elements in solid solution. To determine the amount of retained antimony, calcium, aluminum or tin upon rapid quenching, the cell parameters of α-Pb phase are presented in table (1). The particle size for Pb-Sb-Sn alloy 42 nm is smaller than that for Pb and Egyptian grid battery whereas the minimum particle size was for Pb-Sb-Sn-Ca-Al as show in table 1 and agreement with its curve where the decrease in particle size lead to peak width increase as well as strain micro segregation. A peak shift toward less angle and lead to breadth of cell parameters and then increasing cell volume because inter-planer distances increasing.
3.2. Number of Atoms Per Unit Cell
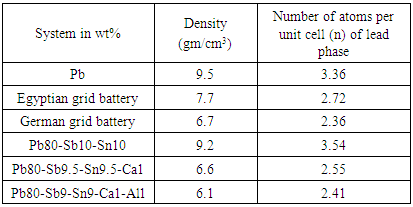
- To come back to the subject of structure determination, the next step after setting up the shape and size of a unit cell is to find the number of atoms per unit cell. To find the number, we use the fact that the volume of the unit cell calculated from the lattice parameters, of α-Pb rich-phase in each melt-spun ribbons of the system used in this study, multiplied by the measured density of each melt-spun ribbons equals the weight of all the atoms in the cell. From the following equation
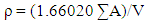 | (1) |
 Where
Where 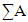 is the sum of the atomic weight of atoms in the unit cell, ρ is the density (gm/cm3), V is the volume of the unit cell (A0)3, and n is the number of atoms per unit cell.
is the sum of the atomic weight of atoms in the unit cell, ρ is the density (gm/cm3), V is the volume of the unit cell (A0)3, and n is the number of atoms per unit cell.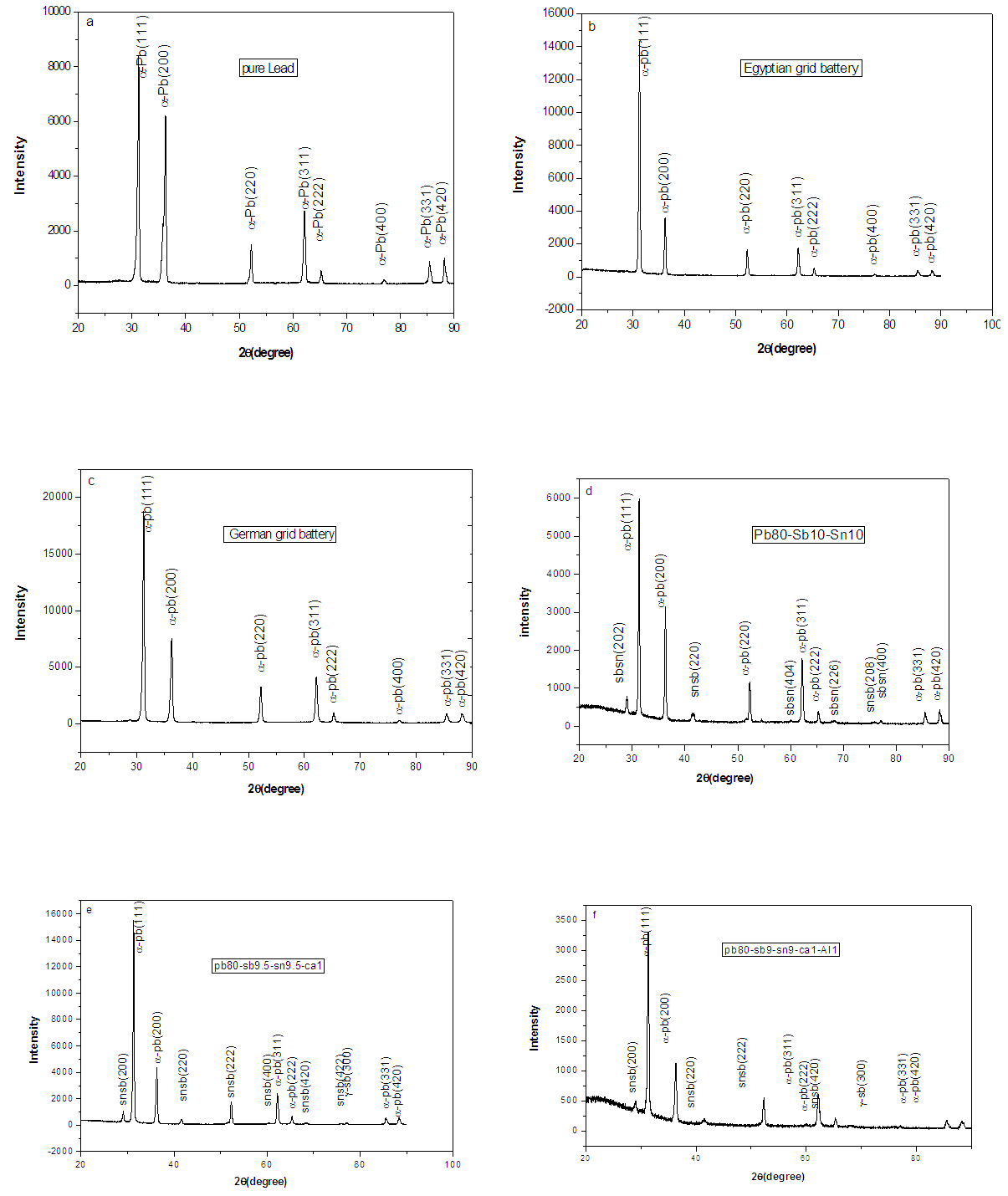 | Figure 1. X-ray diffraction patterns of (a,b,c,d,e,f,g,h and i) melt-spun alloys |
|
|
3.3. Mechanical Properties and Hardness
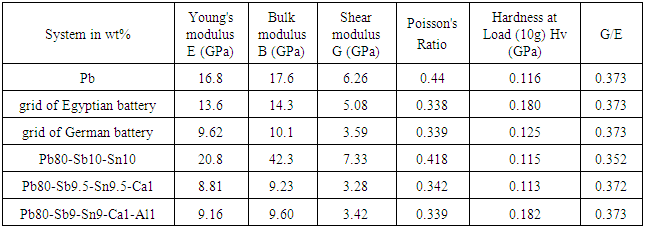
- The calculating values of young's modulus (E) using dynamic resonance method of rapidly solidified systems were studied. The values of the (E) elastic modulus, B bulk modulus, G shear modulus and poison's ratio are listed in table (3). Mechanical properties are associated with the ability of a material to resist mechanical forces. Young’s modulus is the stiffness (the ratio between stress and strain) of a material at the elastic stage of a tensile test. Its intrinsic property which is not affected by the grain size for isotropic materials, but the hardness shows increase with the decrease in grain size according to Hall-Petch [22], where the hardness is engineering property. The values of Young's modulus and other elastic moduli shows slightly dependence on the particle size, it's clear that the maximum Young's modulus for Pb-Sb-Sn was due to strengthen by Sb addition and presence a (SbSn) IMC which specialize with high resistance to plastic deformation. Hardness increases with decreasing particle size. The German battery grid had low fragility which was clearly from the harness and Young's modulus values.
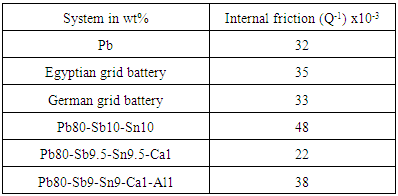
3.4. Internal Friction
- The internal friction is usually applied to the broader study of damping from all possible causes. Internal friction measurement is one of sensitive methods that are suitable to investigating micro structural changes and defect motion, such as atomic diffusion, dislocation activity and grain boundary sliding. Internal friction measurements have been quite fruitful for learning the behaviour of rapidly quenched ribbons from melt. It is one of the important characteristics which are indirectly related to their elastic properties. The free vibration is based on the measurement of the decay in amplitude of vibration during free vibration. The internal friction is obtained by [23]:
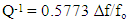 | (2) |
3.5. Electrical and Transport Properties
- The value of electrical conductivity σ according to the quantum theory is:
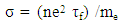 | (3) |
 and Fermi velocity in the same table show a behavior related to the resistivity values, and the changing in Fermi energy values was due to composition changing. The German battery grid is lower in electric resistance stability at higher temperatures as show in temperature Coefficient resistivity.
and Fermi velocity in the same table show a behavior related to the resistivity values, and the changing in Fermi energy values was due to composition changing. The German battery grid is lower in electric resistance stability at higher temperatures as show in temperature Coefficient resistivity.
|
|
|
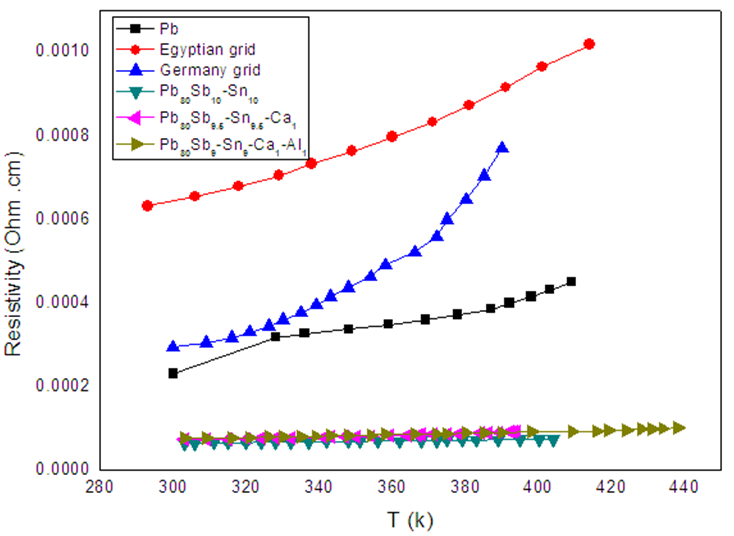 | Figure 2. Electrical resistivity versus temperature for melt-spun ribbons |
4. Conclusions
- The present experimental analysis permits the following conclusion to be drawn:It is apparent from our experience there is a great benefit to be used melt-spinning technique, which have proved to be a powerful techniques in numerous commercial melt-spun ribbon alloy systems. There are indications that the antimony rich precipitates in the structure undergo some degree of dissolution, caused by the rapidly solidified processing. The microstructural changes caused by rapidly solidified in the structure of the material strengthens the material, due to strengthen of the original precipitate structure through the crystallization of the material. It was also found that Pb80-Sb9.5-Sn9.5-Ca1 melt-spun alloy can be used to make ribbon alloys battery grids used for lead storage batteries. A rapidly solidified ternary Pb-Sb-Sn alloy is suitable alloy for storage battery applications in addition it's improved relatively when we adding 1%Ca to it, but not when we adding 1%Al. The use of melt-spinning technique allows an increase in the thickness of important grid member with high quality. It can also use to improve battery longevity. It is clearly seen that the Egyptian battery grid has fragility through the harness and Young's modulus values compared with German battery grid, but it's lower in electric resistance stability at higher temperatures.
ACKNOWLEDGEMENTS
- The author is grateful to Professor Mustafa Kamal, Head of Metal Physics Laboratory, Physics Department, Faculty of Science, Mansoura University, Mansoura, Egypt, for his encouragement and cooperation. Thanks go to Prof. Dr. Abu Bakr El-Bediwi Prof in Metal Physics Laboratory, Physics Department, Faculty of Science, Mansoura University, Mansoura, Egypt, for his guidance and cooperation. Thanks go to Prof. Dr. Rizk El-Sayed Shalaby Prof in Metal Physics Laboratory, Physics Department, Faculty of Science, Mansoura University, Mansoura, Egypt, for his guidance and cooperation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML