-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2016; 6(1): 19-34
doi:10.5923/j.materials.20160601.02

Evaluating the Efficiency of Basalt and Glass Fibres on Resisting the Alkaline, Acid, and Thermal Environments
Sami Elshafie , Gareth Whittleston
Department of Civil Engineering, University of Bolton, Greater Manchester, UK
Correspondence to: Sami Elshafie , Department of Civil Engineering, University of Bolton, Greater Manchester, UK.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Over the past few years, basalt fibre has been used extensively as a reinforcement material in the construction industry, and close attention has been paid to its qualities, especially its chemical and thermal resistance. In view of the significance of basalt fibre as a strengthening material, and to evaluate the long-term durability of basalt fibre, four different experimental works were performed, namely: resistance to alkalinity and acid environment tests, thermal resistance tests on fibres, measuring weight change of fibres, and observing the microstructure and element change of fibres using the Scanning Electron Microscope (SEM) and Energy Dispersive X-ray (EDX). In the past, there have been few studies on the chemical and thermal resistance of basalt fibres, most researchers focused on the effect of the strongest alkaline and acid chemicals such as Hydrochloric acid (HCl), and Sodium hydroxide (NaOH) as well as water on basalt fibres, but paid little attention to the effect of different alkaline and acid chemicals containing different pH levels. This research aims to fill this gap in the literature by evaluating the efficiency of basalt fibres in resisting different strong and weak alkaline and acid chemicals containing different pH levels, and also evaluates the capability of basalt fibres to resist several thermal degrees. For comparison purposes, glass fibres were also tested. The results showed that basalt fibres were more capable of restoring their strength than glass fibres when immersed in different alkaline and acid environments, and were more capable of resisting high heat, in particular resisting temperatures of 300° and 500°. Whereas glass fibres were more capable of resisting the low heat, in particular resisting temperatures of 50° and 100° better than basalt fibres. In this context, the chemical and thermal resistance of basalt fibres becomes an important parameter as a strengthening material for the construction industry.
Keywords: Basalt Fibre, Glass Fibre, Chemical and Thermal Resistance
Cite this paper: Sami Elshafie , Gareth Whittleston , Evaluating the Efficiency of Basalt and Glass Fibres on Resisting the Alkaline, Acid, and Thermal Environments, American Journal of Materials Science, Vol. 6 No. 1, 2016, pp. 19-34. doi: 10.5923/j.materials.20160601.02.
Article Outline
1. Introduction
- Basalt fiber resistance to aggressive media acid, alkaline or thermal is determined by the strength change after some time of exposure in these media. Chemical resistance of basalt fibres depends mainly upon their chemical composition, nature of the aggressive medium, and temperature and time influence on the fiber [22]. In addition, the rate of silicion, aluminum, calcium, and iron oxide in the basalt composition is of high importance. In particular, the presence of the iron oxide in the silicate carcass of the basalt fibre imparts to them higher chemical and thermal resistance when compared with glass fibres [22]. In general, there is no material that is naturally durable because the material’s interaction with the environment changes the properties of the materials. Therefore, the addition of basalt fibres to concrete has a significant effect on the properties of the basalt fibre and, subsequently, it is essential to develop a concrete reinforced by basalt fibres which can resist various in-service environments. In particular, in-service durability is defined as the ability of fibres to resist chemical attack, thermal heat and abrasion while maintaining its desired properties [23]. And Civil Engineers have traditionally focused on enhancing the strength characteristics of the material. However, in recent years, the civil engineering industry has started to view fibres from a different perspective, with an emphasis on the whole-life cycle of the structure, focusing mainly on enhancing the fibres and concrete durability. Hence the durability of fibres is now viewed as equally important as its mechanical properties. [24-26]. Since their occurrence, basalt fibres demonstrated higher alkali resistance compared with majority of glass fibres. This feature has specified the attempts of using them as a reinforcement material of Portland cement concrete.
2. Research Methodology
- To investigate the chemical durability and performance at the cement basalt interface, several types of experimental tests were undertaken. These included; measuring fibres’ weight change, determining the tensile strength of fibres after being boiled in different alkaline and acidic environments, and measuring the thermal resistance of fibres. Surface studies were also carried out by scanning electron microscopy, while microanalysis with complementary X-ray diffraction analysis (SEM/EDS) was also used to ascertain the durability of basalt fibre. The resistance to the alkalinity and acidic environment tests were carried out by boiling the basalt fibres in different alkaline and acids for 1, 5 and 24 hours. Three alkaline chemicals were used (KOH, NaOH, NH3) and three acids (HCl, H2SO4, CH3COOH). The concentrations of the chemicals used were 2 Mol/Litre. The durability test was conducted in accordance with BS EN ISO 175:2010 [17] and similarly to research undertaken by [7, 8, 14], whereby the basalt and glass fibres were boiled in the chemical solutions for 24 hours, and the initial and final mass of the fibres was determined. After boiling and measuring the mass change, the tensile strength of the fibre was then measured. Similarly, the thermal resistance of fibres was performed by heating basalt fibres in a preheated oven at different degrees (50°, 100°, 300° and 500°) for 1, 3 and 6 hours, and the tensile strength was then measured. After each treatment, the tensile strength of basalt fibres was tested by a single fibre testing machine (INSTRON 3369 Dual Column Testing Systems for Tensile, Compression, Flexure testing) [1] in accordance with ISO 5079:1995 [2]. Using a 5kN capacity, tensile load was applied on the fibres at 20mm clamp distance under a constant tensile speed of 3000 mm/min. Finally, the Scanning Electron Microscope (SEM) and energy dispersive x-ray (EDS) were used to monitor the surface shape changes in fibres after being subjected to different chemical and thermal environments, as well as to determine the element change of fibres. The same specimens analysed for the (SEM) are also analysed for (EDS) using 4kV energy. All specimens were coated by carbon, and the results were taken from an average of several fibres. Glass fibres were also tested for comparison purposes.
3. Results and Discussion
3.1. Weight Change of Fibres
- To understand the durability of basalt fibres, a durability test to determine the weight change of basalt fibre boiled in different chemical solutions was conducted. The durability test was conducted by boiling basalt and glass fibres in different chemical solutions: (KOH, NaOH, CH3COOH, HCl, H2SO4, NH3) for 24 hours, and the initial and final mass of the fibres was determined. All results are presented in figure 1.
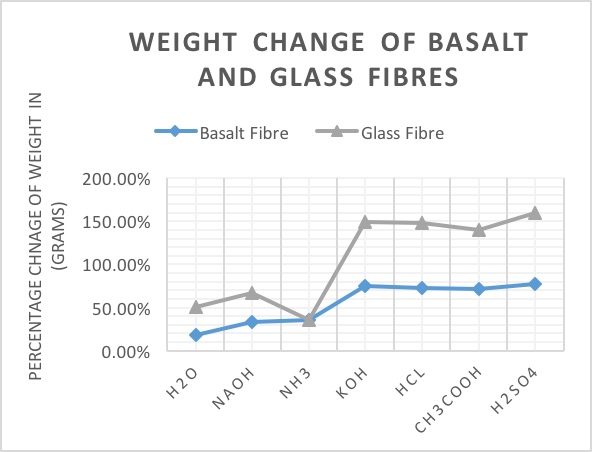 | Figure 1. Weight Change of Basalt and Glass Fibres |
 | (1) |
3.2. Acid Resistance
- An acid is a substance that donates hydrogen ions (Chemical symbol H+). When dissolved in cement or water, there are more hydrogen ions than hydroxide ions in the solution, and as a result the hydroxide ions will move. When mixed with fibres, metal ions on the fibre surface are replaced by hydrogen ions from the acid. Once exposed to acid mediums, the fibres containing alkalis (Na+, K+, Ca2+, Al3+) become prone to chemical attack [5]. An ion exchange mechanism between the fibres and the acid solution starts and is shown by equation (2).
 | (2) |
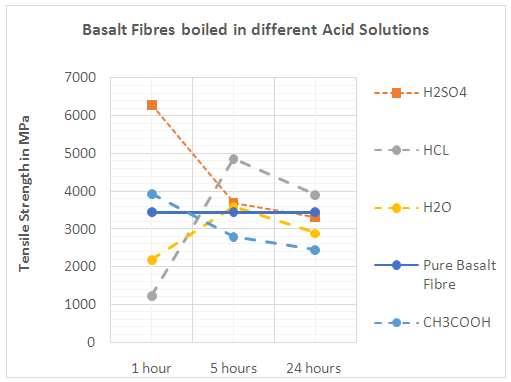 | Figure 2. Basalt Fibres boiled in different Acid Solutions |
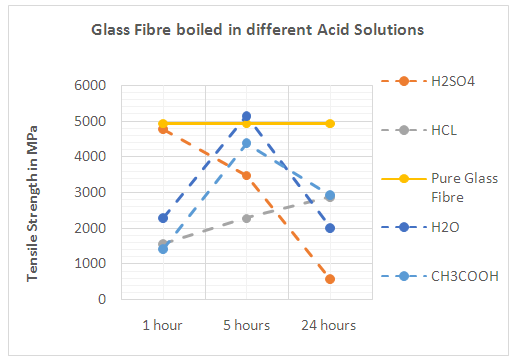 | Figure 3. Glass Fibres boiled in different Acid Solutions |
|
|
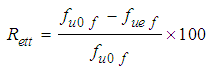 | (3) |
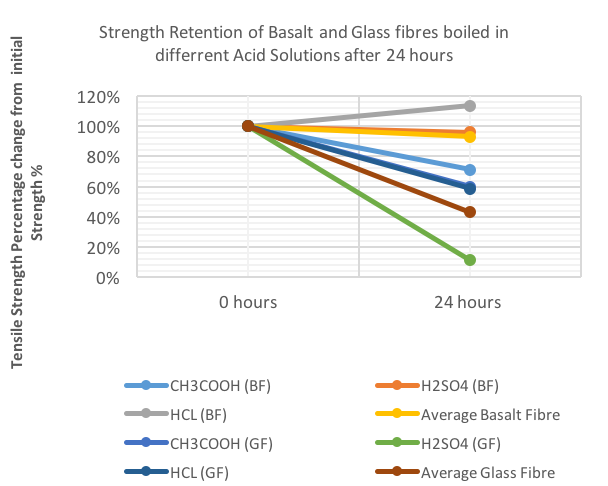 | Figure 4. Strength Retention of Basalt and Glass Fibres boiled in different acid solutions |
3.3. Alkaline Resistance
- An alkaline is a substance that donates hydroxide ions (Chemical symbol OH+) and has a pH level of more than 7. When dissolved in cement or water, there are more hydroxide ions than in the hydrogen ions solution, as a result of the move of the hydroxide ions. An example of an alkaline is NaOH which gives Na+(aq) and OH–(aq) ions in water. When mixed with fibre, a corrosion layer is formed on the surface of the fibre, see picture 17 basalt fibre boiled in NaOH, and the chemical composition of the fibre surface starts to change due to its reaction with an alkaline medium. According to [6] alkali treatment leads to a decrease of sodium, potassium, aluminium and silicon (Na+, K+, Si+, Al3+) on the surface of all basalt fibres. In the alkaline solution, an alkaline attacks the silica network directly and the hydroxide ions of the alkali breaks the Si-O-Si linkage which is shown in Equation (4)
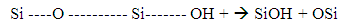 | (4) |
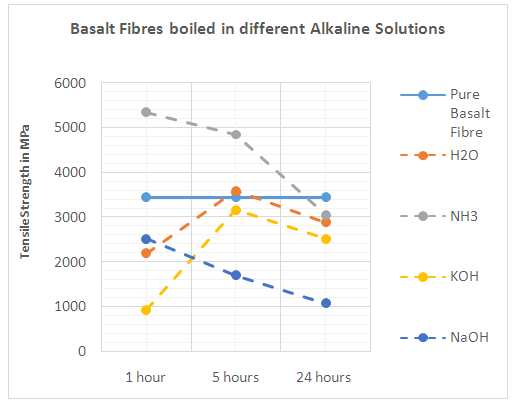 | Figure 5. Basalt fibres boiled in different alkaline solutions |
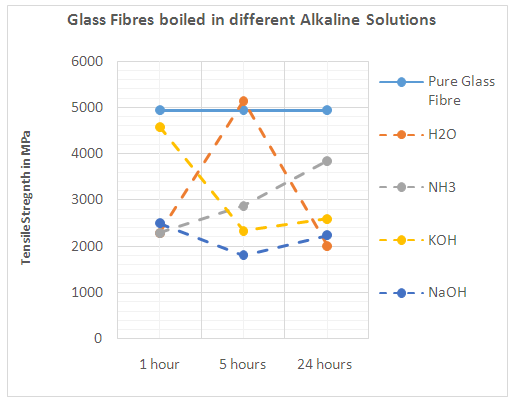 | Figure 6. Glass fibres boiled in different alkaline solutions |
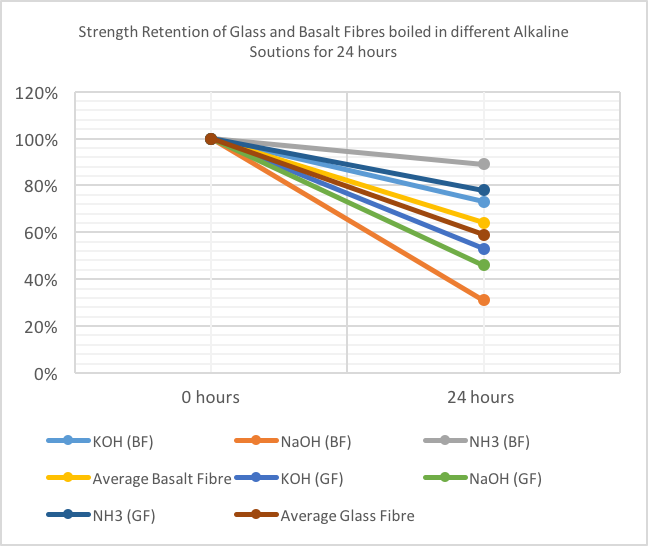 | Figure 7. Strength Retention of Basalt and Glass Fibres boiled in different alkaline solutions |
3.4. Comparison between Fibers Resistance to the Acid and Alkaline Solutions
- Basalt fibre resistance to an aggressive media (acid or alkaline) is determined by the strength change of the fibre after some time of exposure in the media. The ratio of silicon, calcium, magnesium and in the microstructure of the basalt fibre, is of high importance, in particular the chemical interaction between the fibre surface and the chemical environment. In determining if basalt and glass fibres are more capable of resisting the alkaline environments or the acid environments, figures 8 and 9 were drawn. Figure 8 shows the results obtained for boiling basalt fibres in different high and low acid and alkaline solutions as well as the average tensile strength of the basalt fibres in both types of solutions, while figure 9 shows the same attributes using glass fibre.From Figure 8, it can be seen that after 24 hours of boiling fibres in different solution, the values of the tensile strength reduced significantly, which highlights the presence of hydrogen and hydroxide ions, which reduced the tensile strength of the fibres. The reduction values ranged from the highest after immersing basalt fibres in NaOH, to the lowest after immersing fibres in HCL. This result was expected as HCL is a strong acid with a pH level of 0, and NaOH is a strong alkaline with a pH level of 14. On average, basalt fibre performed marginally much better in resisting the acid environment in comparison to resisting the alkaline environment. In contrast, the same result was achieved by [7] as he discovered that the strength of the basalt fibre remains approximately unchanged after being boiled in an acid medium, while the basalt fibres strength declines gradually when boiled in an alkaline medium. Although basalt fibre performed much better in resisting the acid more than the alkaline, its resistance to alkaline remained excellent as highlighted in the previous section. Those results confirmed the results obtained from the first part of the experimental work carried out in this study that highlighted the slight reduction in the tensile strength value by acid chemicals, and an average reduction by the alkaline chemicals.
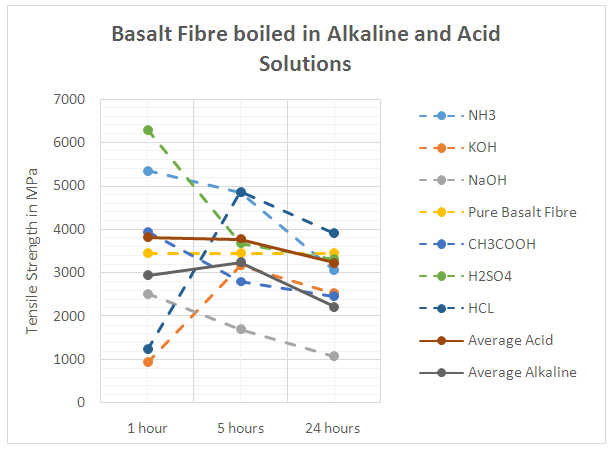 | Figure 8. Performance of Basalt Fibres in different Alkaline and Acid Solutions |
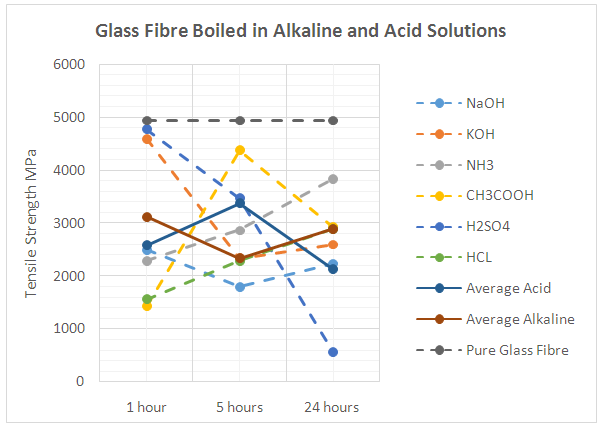 | Figure 9. Performance of Glass Fibres in different Alkaline and Acid Solutions |
3.5. Thermal Resistance of Fibres
- When subjecting fibres to elevated temperature, the microstructure of the fibres degrades at different rates depending on the type of the fibre, atmospheric conditions and time of exposure. Fibres can normally resist heat above their normal operating temperatures, but elevated heat will begin to degrade the fibre. This degradation has the effect of reducing the tensile properties of the fibre and ultimately destroying its integrity [9].To investigate the thermal stability of basalt fibres, the tensile strength of basalt fibres after they were subjected to elevated temperatures (50°, 100°, 300° and 500°) were measured after heating fibres for 1, 3 and 6 hours respectively. The thermal stability of glass fibres were also measured for comparison. Figure 10 shows the basalt fibres’ tensile strength variation after heating fibres for 1, 3 and 6 hours at different temperatures. Before high temperature treatment, the tensile strength of pure basalt fibres were measured. From figure 10 it can be seen that, when basalt fibres were heated for 1 hour at different temperatures, there were no significant strength variation changes in the initial stage, but they then showed some differences after longer periods. When the heat was kept for 3 hours, at low temperatures (50°, 100°) the tensile strength of basalt fibre reduced significantly, whereas at higher temperatures (300°, 500°) the strength of the fibres reduced slightly. When the heating time increased to 6 hours, the strength of the fibres heated at different temperatures remained approximately unchanged. Basalt fibres had better strength retention at high temperatures (300°, 500°) than heating fibres at low temperatures (50°, 100°).
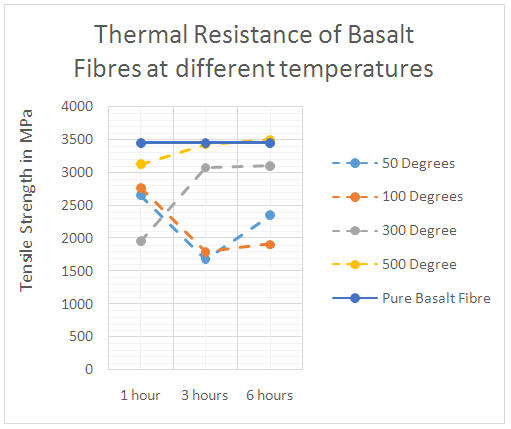 | Figure 10. Thermal resistance of basalt fibres at different temperatures |
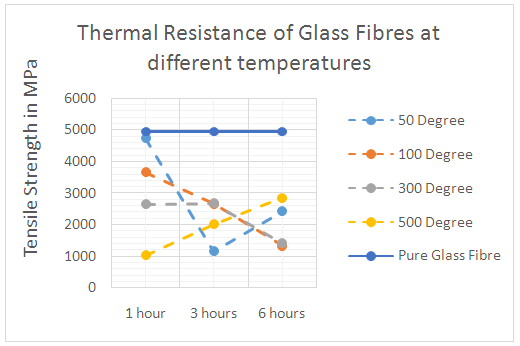 | Figure 11. Thermal resistance of Glass fibres at different temperatures |
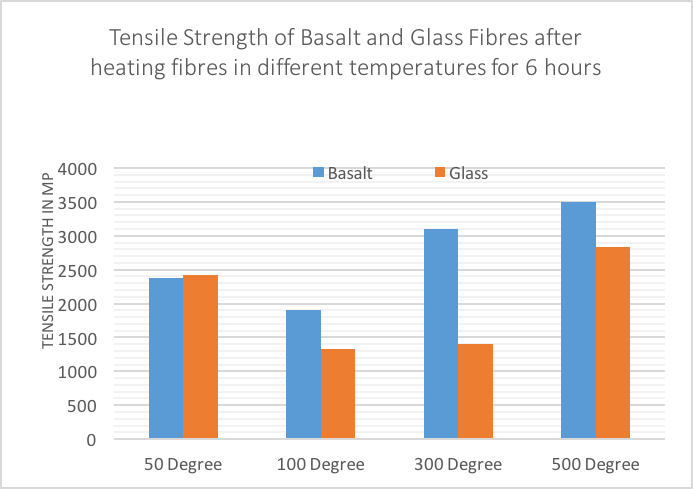 | Figure 12. Tensile Strength of basalt and glass fibres after heating fibres for 6 hours |
3.6. Fibers Surface Observation
- Figures 13 to 20 show the SEM after basalt and glass fibres were boiled in three chemicals (Water, NaOH, HCl) for 24 hours. The appearance of basalt and glass fibres boiled in water and in the strong acid HCl change slightly compared to their original shape. As can be seen from figure 15 and 16, the presence of the defects on the surface of basalt fibres boiled in HCl is much less than glass fibres. The basalt fibres seem to be more compacted and smoother in comparison to the glass fibres. Although the glass fibres boiled in the HCl have a larger fibre diameter in comparison to basalt fibres boiled in the same solution, basalt fibres seem to have less voids, as can be seen from figures 17 to 18. The remaining shape of basalt fibres confirms the brittleness of basalt fibres in the acid environment. The same results were achieved by [7] as the researcher observed slight surface change to basalt fibres when immersed in the acid solution HCl. The combination of the basalt fibres’ compaction and interaction with the matrix made its structure stronger to resist the acid environment.
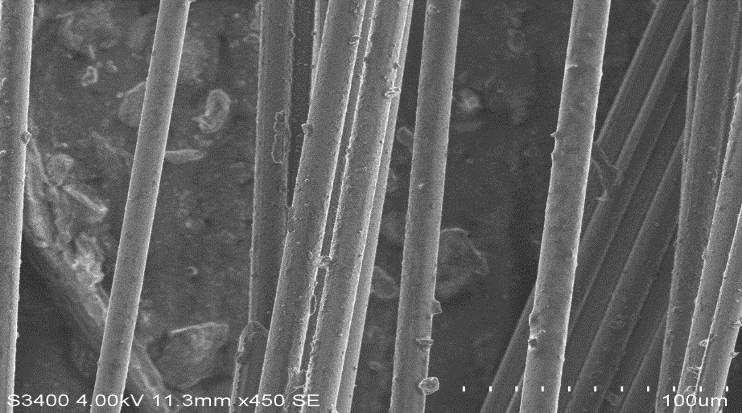 | Figure 13. Pure Basalt Fibre |
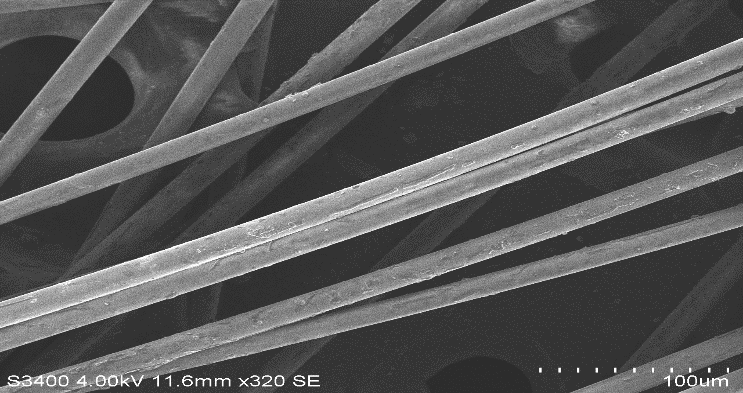 | Figure 14. Pure Glass Fibre |
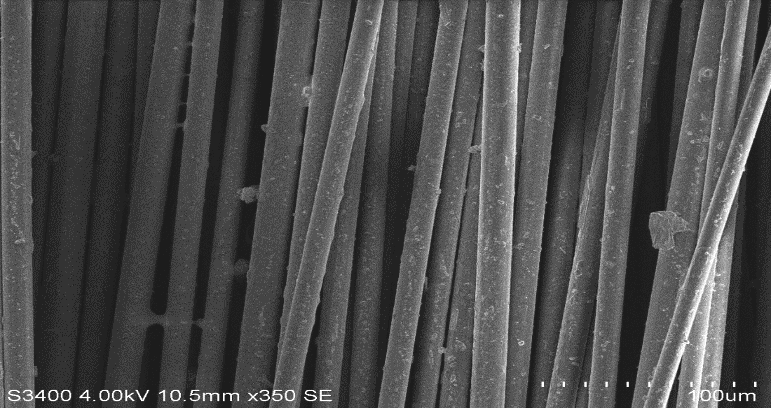 | Figure 15. Basalt fibre boiled in HCl for 24 hours |
 | Figure 16. Glass fibre boiled in HCl for 24 hours |
 | Figure 17. Basalt fibre boiled in NaOH for 24 hours |
 | Figure 18. Glass fibre boiled in NaOH for 24 hours |
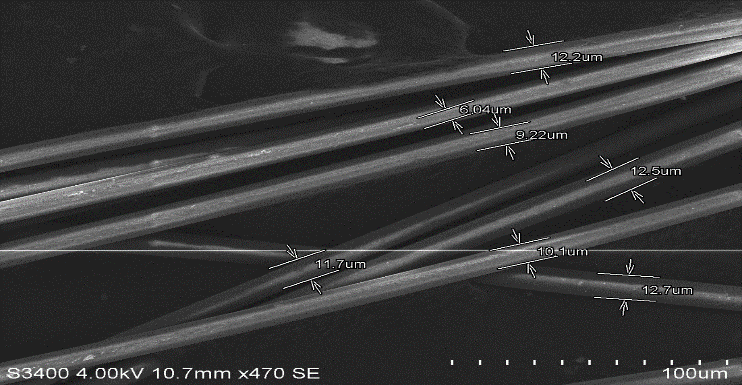 | Figure 19. Basalt fibre boiled in Water for 24 hours |
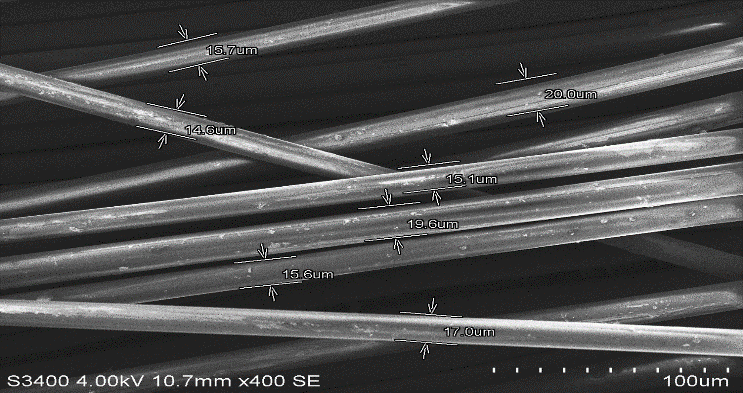 | Figure 20. Glass fibre boiled in Water for 24 hours |
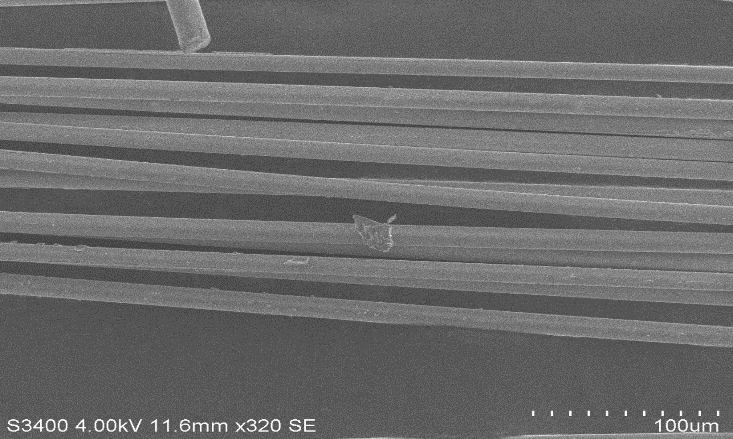 | Figure 21. Basalt fibre heated at 500° |
 | Figure 22. Glass fibre heated at 500° |
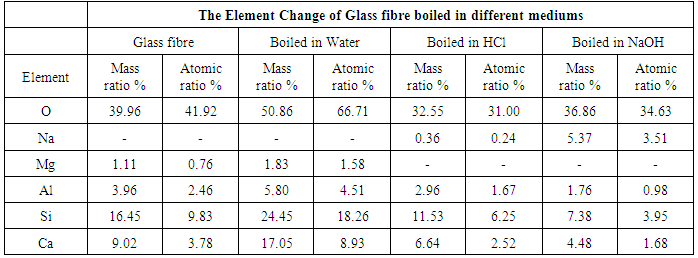
3.7. Element Change of Basalt and Glass Fibers
- In recent years, transition metal oxides have been studied with a view to understanding their effect on the fibres’ strength. Therefore, the element content of basalt fibre after being boiled in water, HCl and NaOH were analysed using EDS and the results are shown in table 1. The EDS analysis showed that basalt fibre consisted of a number of oxides which have an essential impact on its properties. Dominant O2 represented around 38% of its weight, followed by carbon (C) 26%, Silicon (Si) around 15%, and other oxides form just below 5% of its weight. Chemical properties in, particular Si, influences the mechanical properties of the fibres, especially when it reacts with an acid or an alkaline solution. From table 1 it can be seen that the element change of basalt fibre boiled in water for 24 hours stays almost the same as the element of the original fibre. In contrast, some elements’ content, such as Ca, K, Ti and Fe, decreased slightly, but the content of carbon (C) increased rapidly. The slight change in the content of basalt fibre boiled in water confirms the excellent resistance of basalt fibre to water. The element changes in acid and alkaline are different. In acid, the content of metal elements C and Ti decreased rapidly. However there is a high increase in the content of Silicon (Si). This is due to the replacement of the metal atoms by hydrogen ions (H+) and Chloride (Cl) from the acid [7]. This replacement will have an impact on the SI – O – Si structure, as a result increasing the content of Si and improving the fibre strength. This result was also achieved in previous section of this research-using figure 4, as the basalt fibre gained tensile strength when immersed in HCl compared to the original basalt fibre strength. In an alkaline medium, the decrease of the Si element is observed when boiled in NaOH and notably the Na increases in the basalt fibre boiled in NaOH. This is due to the network being destroyed directly by OH. The reaction can be expressed as in equation (5):
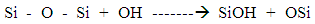 | (5) |
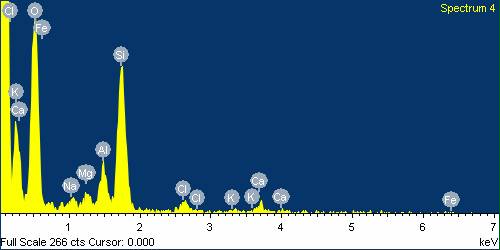 | Figure 23. EDS analysis for Basalt Fibre boiled in HCl for 24 hours |
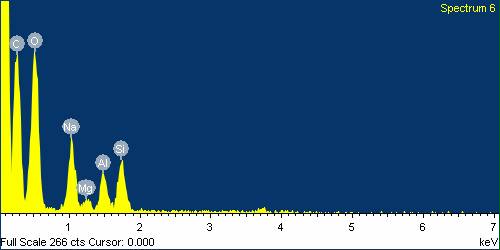 | Figure 24. EDS analysis for Basalt Fibre boiled in NaOH for 24 hours |
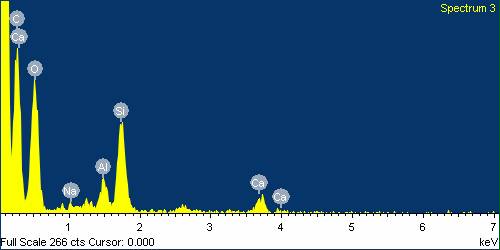 | Figure 25. EDS analysis for Glass Fibre boiled in HCl for 24 hours |
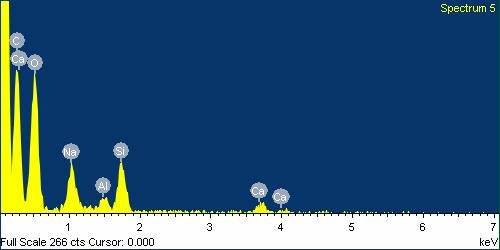 | Figure 26. EDS analysis for Glass Fibre boiled in NaOH for 24 hours |
4. Conclusions
- In this paper, a new method of evaluating the efficiency of fibres to resist the alkaline, acid, and thermal environments is proposed. The proposed method is applied by boiling basalt and glass fibres in different strong and weak alkaline and acid chemicals containing different pH levels, as well as subjecting fibres to several thermal temperatures. This comprehensive investigation of fibres’ durability has outlined the capability of fibres to resist several chemical environments, and shown the ultimate benefits that can enhance composite durability. Basalt fibres were tested to resist temperatures achieved during typical cement hydration which is approximately 74°C according to [18], also being tested at fire level temperatures, as concrete begins to degrade above 300°C [19]. Another issue occurs when constructing concrete bridges. Due to carbonation, the alkalinity of the pore fluid drops from a pH value exceeding 12.6 to a value about of 8.0 [20]. On the other hand, concrete used in waste water treatment such as waste water tanks is exposed to harsh chemicals such as acids, salts and sulphates, ph level between 2 – 6, and if these chemicals have had a chance to get inside the concrete, they will cause damages [21]. As a result, fibres were tested against several heat degrees and different chemicals containing different ph levels. The results show that basalt fibres seem to have gained less weight than glass fibres when boiled in alkaline and acid solutions. In addition, basalt fibres have performed much better in resisting the acid environment than the alkaline environment. On the other hand, the average result achieved for glass fibre to resist alkaline solutions is higher than the average result achieved for glass fibre to resist the acid environment. The paper also confirmed that basalt fibres are more capable of restoring their strength than glass fibres, more capable of resisting high heat, and can maintain their strength better than glass fibres, in particular resisting temperatures of 300° and 500°. In contrast, glass fibres are more capable of resisting low heat, in particular resisting temperatures of 50° and 100° better than basalt fibres. In addition, the scanning electron microscope showed that basalt fibres seem to be more compacted and smoother when boiled in HCl compared to glass fibre, and a film was formed on the surface of the basalt and glass fibres when immersed in NaOH. There was a variation in the element change of basalt and glass fibres when boiled in different alkaline and acid solutions, especially for the glass fibre as it lost many of its original elements.
ACKNOWLEDGEMENTS
- I would like to thank Dr. Gareth Whittleston for his contributions to this study, and David Simmons for supplying the basalt fibres required to do this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML