-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2015; 5(4): 90-95
doi:10.5923/j.materials.20150504.03
Synthesis and Characterization of Polyaniline-Multiwalled Carbon Nanotube Nanocomposites and Its Electrical Percolation Behavior
S. G. Bachhav, D. R. Patil
Nanomaterial Research Laboratory, R.C. Patel Arts, Commerce & Science College, Shirpur, India
Correspondence to: D. R. Patil, Nanomaterial Research Laboratory, R.C. Patel Arts, Commerce & Science College, Shirpur, India.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The nanocomposites of polyaniline (PANI) and carboxylated multi-walled carbon nanotubes (MWCNTs) were synthesized by in situ chemical oxidative polymerization method using HCl as a dopant and Ammonium persulphate (APS) as an oxidant. The MWCNTs were carboxylic functionalized and were ultrasonicated to obtain uniform dispersion within the PANI matrix. Field emission scanning electron microscopy (FE-SEM) was used to characterize the morphology of the nanocomposites. X-Ray diffraction (XRD), Fourier Transform Infrared (FT-IR) spectroscopy, Raman spectroscopy and UV-Vis spectroscopy were used to characterize the synthesized PANI-MWCNT nanocomposites. It was found that in situ polymerized PANI layer matrix was formed on carboxylated MWCNT and there was uniform dispersion of MWCNTs within the PANI matrix with significant interaction between PANI and MWCNTs. The electrical conductivity of composites at room temperature was measured and their percolation threshold briefly discussed.
Keywords: Polyaniline, Carbon nanotubes, in situ polymerization, Electrical conductivity
Cite this paper: S. G. Bachhav, D. R. Patil, Synthesis and Characterization of Polyaniline-Multiwalled Carbon Nanotube Nanocomposites and Its Electrical Percolation Behavior, American Journal of Materials Science, Vol. 5 No. 4, 2015, pp. 90-95. doi: 10.5923/j.materials.20150504.03.
Article Outline
1. Introduction
- A polymer-Carbon nanotubes (CNTs) composite is a combination of polymer matrix with CNTs that possess properties that are unique and cannot be obtained with each material acting alone. CNTs with unique electrical, thermal and mechanical properties has become a potential candidate for wide range of applications in nanoscience and nanotechnology, suitable to serve as conducting filler in polymer nanocomposites. As carbonaceous nanofillers, CNTs play a very promising role due to their better structural and functional properties such as high aspect ratio, high mechanical strength, and high electrical properties etc. than other fillers [1].The conducting polymers receive much attention because of their wide application range. Among the several conducting polymers, Polyaniline (PANI) has been studied extensively due to its high electrical conductivity, abundant raw materials, easy synthesis, good environmental stability, cost effectiveness and simple redox chemistry [2]. The PANI-CNT nanocomposites is one of the versatile nanocomposites due to the numerous applications such as Gas sensor [3, 4], Biosensor [5], Supercapacitor [6], Solar cell [7], Fuel cell [8], Corrosion protection [9], etc. Out of several synthesis methods of PANI-CNT nanocomposites reported in literature, in situ polymerization is the most used synthesis method as it enables grafting of polymer molecules on CNT, which leads to the better dispersion coefficient and better interaction between CNT and polymer matrix. Incorporating CNT as fillers into PANI enhance the conductivity of PANI nanocomposites as CNT could have much higher electrical conductivity than PANI. The CNT could behave as conducting bridge between conducting domains of PANI thus enhanced the electrical conductivity of PANI [10]. The effect of the functionalized multi-walled carbon nanotubes (f-MWCNTs) content on the conductivity of PANI-MWCNT composites was studied by many researchers [11, 12]. For low fractions of CNT, the conductivity is mainly determined by the PANI as it is the main constituent. The conductivity of PANI Emeraldine Salt based composites usually increases with CNT content [13, 14]. When an adequate amount of filler is loaded, a ‘‘percolation’’ path of connected fillers forms and allows charge transport through the material. At this critical concentration, called the percolation threshold, the conductivity rapidly increases.In the present work, we report the synthesis of PANI-MWCNT nanocomposites by in situ polymerization. MWCNTs can be covalently functionalized to well disperse and to enable grafting surface. This work was aimed to obtained good dispersion of MWCNTs within PANI matrix to achieve enhancement in electrical properties of the nanocomposites. The percolation behaviour of synthesized nanocomposites was also discussed.
2. Experimental
2.1. Materials
- MWCNTs and Triton X-100 used were received from sigma Aldrich. The aniline monomer used was acquired from Rankem (Mumbai). Ammonium persulphate (APS) was used as oxidizing agents acquired from SDFC (Mumbai). All these reagents used were analytical grade.
2.2. Functionalization of MWCNT
- Surface modification and functionalization of MWCNTs were made by using acid boiling reflux treatment. For this, MWCNTs (0.5 g) were suspended in H2SO4 and HNO3 in a ratio of 3:1v/v to introduce the carboxylic group into the surface of MWCNTs. The suspension was refluxed with vigorous stirring at 80°C for 12 h. After cooling to the room temperature, the mixture was filtered with filter paper. The filtrated solid was then washed thoroughly with DI water until neutral pH, and then washed with ethanol. The collected product was dried in oven at 60°C for 12 h. This product is referred to as functionalized MWCNTs [15].
2.3. Synthesis of PANI-MWCNT Nanocomposites
- The PANI-MWCNT nanocomposites were synthesized by in situ chemical oxidative polymerization. In a typical synthesis experiment, the desired quantity of functionalized MWCNTs was added to 60 ml distilled water. A certain amount of Triton X-100 was added as a surfactant to this mixture so as to disperse the MWCNTs in water. The solution then ultrasonicated over 1 h to form well dispersed MWCNT solution. The 2 ml of aniline monomer was added into this MWCNT solution with constant stirring. After addition of aniline to MWCNT solution, the mixture was again sonicated for 10 min. The 60 ml of 1M HCl was prepared and added to the Aniline-MWCNT solution. The 0.35M APS solution was prepared by dissolving required amount of APS into 60 ml distilled water and was added drop wise to Aniline -MWCNT-HCl mixture solution for 30 min with constant stirring. The stirring was continued for 4 h and then kept standing for overnight to obtained polymerized dark-green precipitate. The polymerized solution was then filtered and washed several times with distilled water until the filtrate became acid free, and then washed with acetone, ethanol and methanol. The precipitate was dried in an oven at 60°C for 12 h to obtain PANI-MWCNT nanocomposite.The six PANI-MWCNT nanocomposite samples were prepared for different amount of functionalized MWCNTs such as 5 mg, 10 mg, 20 mg, 40 mg, 80 mg and 160 mg keeping PANI concentration constant. The % content of MWCNT in PANI matrix was 0.25%, 0.50%, 1%, 2%, 4% and 8%. The pristine PANI sample was also synthesized using the similar method given above except use of MWCNTs.
2.4. Characterization
- The morphology of the synthesized nanocomposites was studied on Hitachi S4800 Type-II Field Emission Scanning Electron Microscopy (FESEM). X-ray diffraction (XRD) patterns were performed on a Bruker D8 Advance diffractometer with Cu Kα radiation. Raman spectra were recorded at room temperature on JY Horiba HR800 Raman spectrometer employing 488 nm Argon laser beam. Fourier transform infrared (FT-IR) spectra were recorded on a Shimadzu IR Affinity spectrophotometer with KBr pellets to ensure functional groups. UV-Vis spectra were recorded on Shimadzu UV-2450 spectrophotometer using quartz cell.
2.5. Conductivity Measurement
- The conductivity of as synthesized composites at room temperature were determined by making the pellets of 15 mm diameter by motorized hydraulic press under identical conditions such as pressure, temperature, time etc. The silver paste contact was made on both sides of pellet and bulk resistance was measured by Keithley 2000 multimeter. The dc conductivity was then determined using the measured resistance and thickness of pellet.
3. Result and Discussion
3.1. Characterization of PANI-MWCNT Nanocomposite
- The FESEM images of pure PANI, PANI-MWCNT and functionalized MWCNTs were shown in Figure 1. The intermingle ropes with smooth surface of functionalized MWCNTs with diameter about 10 nm and lengths up to few μm was observed as shown in Figure 1(a). The pure PANI granular structure with average diameter about 100 nm is observed in the image shown in Figure 1(b). The nanocomposites obtained by grafting PANI on surface of MWCNTs producing thick layer PANI on MWCNT resulted in elongated granular form with diameter in the range of 100-150 nm for 4 wt. % MWCNT loading as observed in Figure 1(c). The same structure with average diameter of 15-20 nm was observed for higher loading of MWCNTs (8 wt%), which revealed that thin layer of PANI deposited on surface of MWCNTs as shown in Figure 1(d).
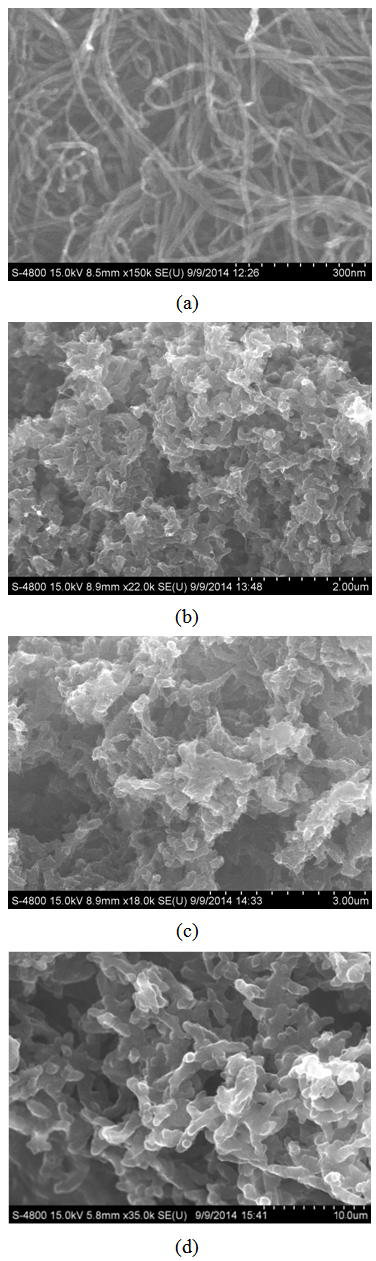 | Figure 1. FE-SEM images of (a)f-MWCNT b) PANI and PANI-MWCNT nanocomposites at MWCNTs contents of (c) 4 wt.% and (d) 8 wt.% |
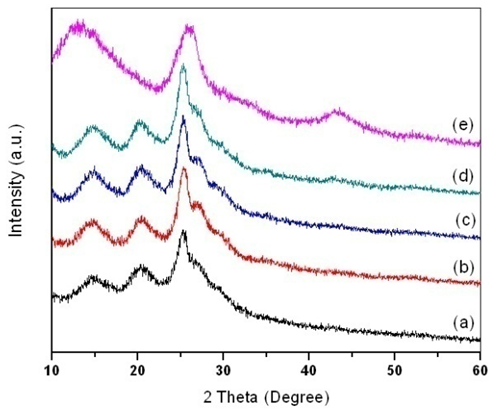 | Figure 2. XRD patterns of (a) PANI, PANI-MWCNT nanocomposites at MWCNT contents of (b) 0.5 wt.%; (c) 4 wt.%; (d) 8 wt.%; (e)f-MWCNT |
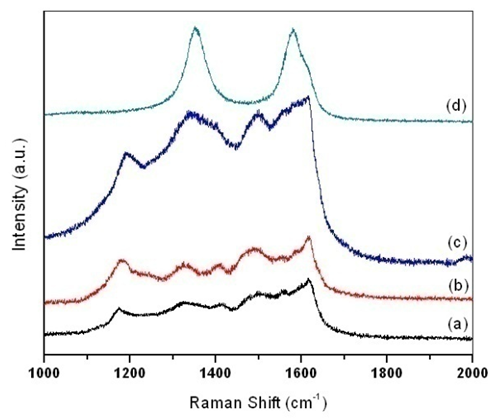 | Figure 3. Raman spectroscopy of (a) pure PANI, PANI-MWCNT nanocomposites at MWCNTs contents of (b) 4 wt. %; (c) 8 wt. % and (d) MWCNT |
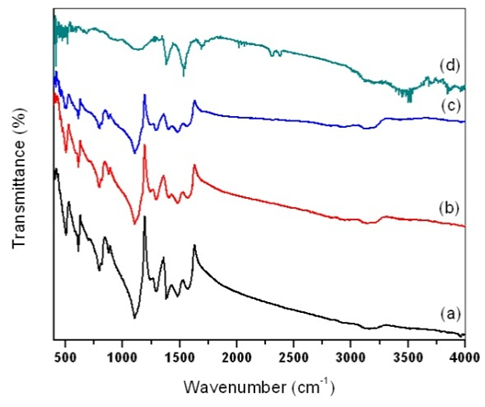 | Figure 4. FT-IR spectrum of (a) pure PANI, PANI-MWCNT nanocomposites at MWCNTs contents of (b) 2 wt. %;(c) 8 wt. % and (d) MWCNT |
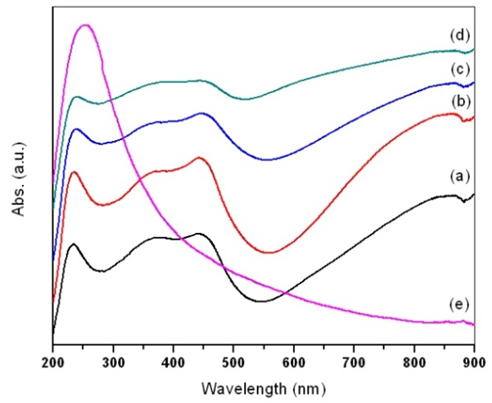 | Figure 5. UV-Visible spectra of (a) pure PANI, PANI-MWCNT nanocomposites at different MWCNTs contents of (b) 0.5 wt %; (c) 1 wt %; (d) 4 wt %; (e) MWCNT |
3.2. Electrical Conductivity
- The curve of dc conductivity at room temperature against MWCNT contents is shown in Figure 6. It was found that conductivity was lowest for pure PANI and increased with content of MWCNT [29]. It was increased slowly for up to 1% MWCNT content but increased rapidly after 1 wt % MWCNT content. The lowest conductivity obtained for pure PANI is 0.17 S/cm and increased from the value 0.22 S/cm to 3.32 S/cm for PANI-MWCNT composites having MWCNT concentration range of 0.25% to 8%. This represents an increase of conductivity nearly twenty times of magnitude as compared with pure PANI, which may be attributed to the synergistic effect between PANI and MWCNT in forming interpenetrating conductive network. The lower conductivity at low filler concentration is due the fact that excessive PANI formed aggregation over the surface MWCNTs, consequently conductive channels formed by MWCNTs are separated from each other. This prevented the transport of effective charge along the conductive network [30] and the conductivity of PANI-MWCNT nanocomposites are contributed by pristine PANI only [31].
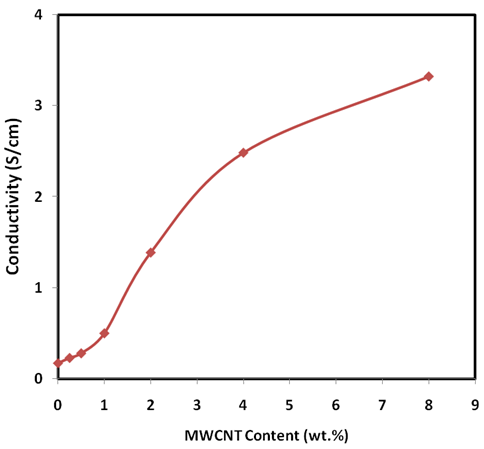 | Figure 6. Room temperature electrical conductivity of PANI-MWCNT nanocomposites versus the weight percentage of MWCNT |
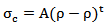 | (1) |
 represents the electrical conductivity of the nanocomposite, A is a constant,
represents the electrical conductivity of the nanocomposite, A is a constant, 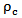 the percolation threshold,
the percolation threshold, 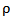 the filler mass fraction, and t the critical exponent for the conductivity. The expression given in Equation (1) is valid for both insulating as well as conductive matrices incorporated with highly conductive fillers. The above equation clearly indicates that as the filler mass fraction increases beyond the percolation threshold, the composite conductivity increases sharply as conductive network paths begin to establish. It can observe that for as synthesized nanocomposites, the percolation threshold occurs at 1 wt % MWCNT content.The filler content is not only the parameter to determine the electrical conductivity of the composite, but the filler aspect ratio is another important conductivity dependent parameter. The conducting fillers such as MWCNTs with high aspect ratio are more effective in forming conductive network and produce a low percolation threshold [34, 35].
the filler mass fraction, and t the critical exponent for the conductivity. The expression given in Equation (1) is valid for both insulating as well as conductive matrices incorporated with highly conductive fillers. The above equation clearly indicates that as the filler mass fraction increases beyond the percolation threshold, the composite conductivity increases sharply as conductive network paths begin to establish. It can observe that for as synthesized nanocomposites, the percolation threshold occurs at 1 wt % MWCNT content.The filler content is not only the parameter to determine the electrical conductivity of the composite, but the filler aspect ratio is another important conductivity dependent parameter. The conducting fillers such as MWCNTs with high aspect ratio are more effective in forming conductive network and produce a low percolation threshold [34, 35].4. Conclusions
- A PANI-MWCNT nanocomposites has been successfully synthesized by in situ oxidation polymerization of aniline monomer in the presence of MWCNT with different content. FE-SEM images confirm that PANI has successfully formed on the surface of MWCNTs. XRD, Raman, FT-IR and UV-vis spectra revealed the incorporation of MWCNT into the PANI matrix. The significant enhancement in conductivity is observed with increasing MWCNT weight percentage and is indicative of percolative character. The percolation threshold of conductivity was occurred at 1 wt % MWCNT content for synthesized PANI-MWCNT nanocomposites.
ACKNOWLEDGMENTS
- The authors are grateful to University Grant Commission (UGC), New Delhi for financial support through minor research project scheme no. F. 47-809/13 (WRO). The authors are also thankful to UGC-DAE Consortium for Scientific Research centre, Indore for providing the characterization facilities and the management of R.C. Patel Educational trust for their continuous encouragement and support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML