-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2015; 5(3): 66-68
doi:10.5923/j.materials.20150503.02
Structural and Optical Investigation of ZnO Nanopowders Synthesized from Zinc Chloride and Zinc Nitrate
N. Srinivasa Rao1, 2, Mandava V. Basaveswara Rao2
1Department of Chemistry, SKBR Govt. Degree College, Macherla, Guntur
2Department of Chemistry, Krishna University, Krishna Dist., Andhra Pradesh
Correspondence to: Mandava V. Basaveswara Rao, Department of Chemistry, Krishna University, Krishna Dist., Andhra Pradesh.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
ZnO Nanopowders were synthesized from Zinc Chloride and Zinc Nitrate by Co-precipitation Method. The synthesized ZnO nanopowders were further characterized using X Ray Diffraction (XRD), Scanning Electron Microscopy (SEM) and Fourier Transform Infrared (FTIR) Spectroscopy. The Structure, Morphology and Optical properties were investigated for each precursor. XRD patterns indicate hexagonal wurtzite structure with high crystallinity. SEM images revealed the morphology of the ZnO Particles. The optical characterizations of ZnO nanoparticles have been carried out using FTIR Spectroscopy. The spectrum shows the characteristic absorption peaks of Zn-O are nearer to 390 cm-1 and also authenticates presence of ZnO. From the analyses of results, it is very clear that precursors used in the present study have played a vital role in surface morphology, structural and optical properties of ZnO nanoparticles.
Keywords: ZnO, Nanopowders, Zinc Chloride, Zinc Nitrate, Co-precipitation, XRD, SEM, FTIR
Cite this paper: N. Srinivasa Rao, Mandava V. Basaveswara Rao, Structural and Optical Investigation of ZnO Nanopowders Synthesized from Zinc Chloride and Zinc Nitrate, American Journal of Materials Science, Vol. 5 No. 3, 2015, pp. 66-68. doi: 10.5923/j.materials.20150503.02.
Article Outline
1. Introduction
- ‘Nanotechnology’ is the new frontier of science and technology around the world working at the scale of individual molecules. In fact, one nanometer (nm) is one billionth of a meter, tens of thousands times smaller than the width of a human hair. There has been a great deal of interest on Zinc Oxide nanostructures lately as seen from the surge of a relevant no of publications. The interest in ZnO is fuelled and fanned by number of prospects in optoelectronic device applications owing to its wide band gap (Eg-3.3eV at 300 K), large bond strength and large exiton binding energy (60meV). Most preferentially among different metal oxide nanoparticles, ZnO nanoparticles have their own importance due to their vast area of applications, e.g., gas sensor, chemical sensor, bio-sensor, cosmetics, storage, optical and electrical devices, window materials for displays, solar cells and drug-delivery. Due to its vast areas of application, various synthesis methods have been employed to grow a variety of ZnO nanostructures, including nanoparticles, nanowires, nanorods, nanotubes, nanobelts, and other complex morphologies (1-7). In the present work, we mainly focus on ZnO nanoparticles synthesized by cost effective and simple co-precipitation method from Zinc Chloride and Zinc Nitrate (7-8).
2. Experimental Procedure
2.1. Materials
- Zinc Chloride (ZnCl2), Zinc Nitrate (Zn(NO3)2), Potassium Hydroxide (KOH) and Ethanol (C2H6O) were purchased and used without further purification.
2.2. Experimental Procedure
- The fabrication of ZnO nanopowders consists basically a jacketed three-neck glass flask and a magnetic stirrer, centrifuge and micro oven. In this experiment 0.4 M aqueous ethanol solution of zinc chloride (Zn(Cl2)2·4H2O) was kept under constant stirring using magnetic stirrer to completely dissolve the Zinc Chloride for one hour and 0.8M aqueous ethanol solution of Potassium Hydroxide (KOH) was also prepared in the same way with stirring of one hour. After complete dissolution of Zinc Chloride, 0.8 M KOH aqueous solutions were added under high speed constant stirring drop by drop (slowly for 45 min) touching the walls of the vessel. The reaction was allowed to proceed for 2 hrs after complete addition of potassium hydroxide. The beaker was sealed at this condition and the solution was allowed to settle for overnight. Further, the supernatant solution was separated carefully. The remaining solution was centrifuged for 10 min and the precipitate was removed. Thus, precipitated ZnO nanoparticles were cleaned three times with deionised water and ethanol to remove the by products which were bound with the nanoparticles and then dried in air atmosphere at about 60°C. During drying, Zn(OH)2 is completely converted in to ZnO.In a similar manner ZnO nanoparticles were synthesized from Zinc Nitrate. The two samples were further characterized for their optical and nanostructural properties.
3. Characterization
- The synthesized particles were primarily investigated by XRD for structural and phase analysis. The morphology was obtained by scanning electron microscope (SEM). The optical properties were investigated using Fourier Transform Infra red (FTIR) spectrum. The presence or absence of the various vibrational modes present in the synthesized nanoparticles was investigated.
4. Results and Discussions
4.1. XRD (X Ray Diffraction)
- ZnO Nanopowders with different grain sizes can be obtained from different precursors. Fig 1.1 & Fig 1.2 represent the XRD patterns of ZnO Nanopowders synthesized from Zinc Chloride and Zinc Nitrate respectively. The XRD data was recorded using CuKβ radiation. The intensity data was collected over a 2θ range of 20-80°. These XRD patterns show much sharper peaks. In the Fig1.1 the distinctive ZnO peaks at 31.51, 34.658, 36.453, 47.74, 56.78, 62.99, 66.38, 68.06, 69.16 and 79.96 respectively. In the Fig 1.2 the distinctive ZnO peaks at 31.946, 34.631, 36.448, 47.755, 56.769, 62.928, 66.45, 68.09, 69.19, 77.17 and 88.56 respectively.
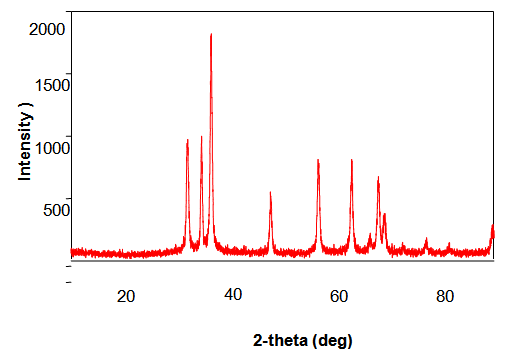 | Figure 1.1. XRD pattern of ZnO Nanopowders synthesized From Zinc Chloride |
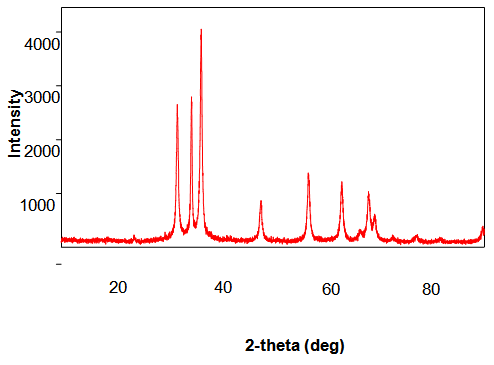 | Figure 1.2. XRD pattern of ZnO Nanoparticle synthesized from Zinc Nitrate |
4.2. SEM (Scanning Electron Microscopy)
- The morphology of ZnO nanoparticles were studied from SEM pictures. Fig 2.1 & Fig 2.2 represent the SEM micrographs of ZnO nanoparticles observed at different magnifications. The high resolution SEM images show the presence of nanoparticles. The SEM images represent the agglomeration of particles and also with narrow particle size distribution. It is also be seen that the samples synthesized were nanoflakes which were turned to the particles when observed at different magnifications.
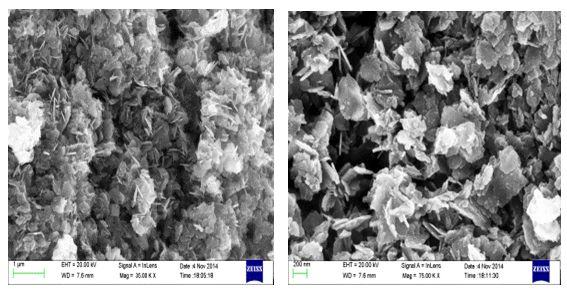 | Figure 2.1. SEM images of ZnO Nanoparticles observed at different magnifications synthesized using Zinc Chloride |
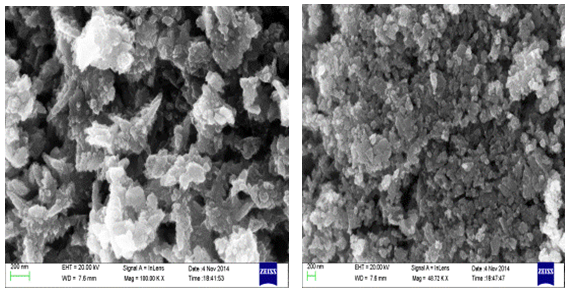 | Figure 2.2. SEM images of ZnO Nanoparticles observed at different magnifications synthesized using Zinc Nitrate |
4.3. FTIR (Fourier Transform Infrared) Spectroscopy
- FTIR spectroscopy is performed in order to quickly establish the presence or absence of the various vibrational modes present in synthesized particles. The Fig. 3.1 & Fig 3.2. Shows the FTIR spectrum of the ZnO nanoparticles synthesized from Zinc Chloride and Zinc Nitrate acquired in the range of 500-4000 cm-1. Various modes of vibration are observed at different regions of FTIR spectrum. The peaks at 4,000 and 500 cm–1 observed in the spectrum indicate the presence of –OH and C=O residues which may be due to precursors used in reaction.
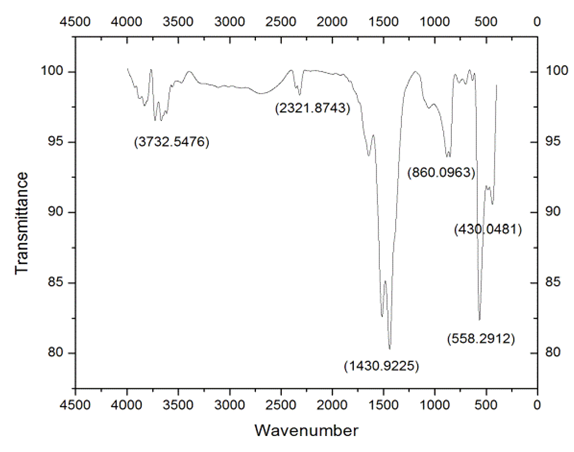 | Figure 3.1. FTIR Spectrum ZnO Nanopowders synthesized From Zinc Chloride |
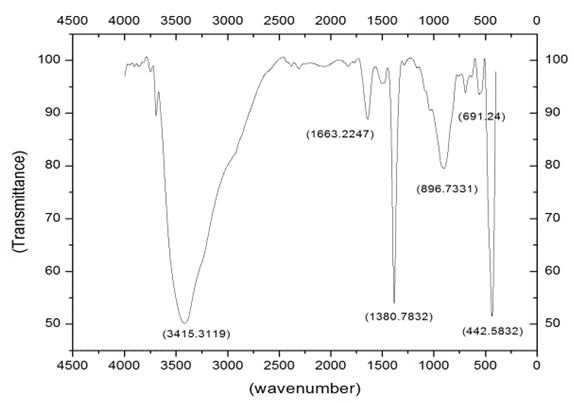 | Figure 3.2. FTIR spectrum ZnO Nanopowders synthesized From Zinc Nitrate |
5. Conclusions
- Zinc oxide nanoparticles were synthesized by a cost effective and simple Co-precipitation method from Zinc Chloride and Zinc Nitrate respectively. The effect of precursors on structural and optical properties of ZnO nanoparticles was investigated. The XRD patterns represent the wurtzite phase of ZnO nanoparticles. Nanoparticles with an average size of 21.59 nm & 19.6 nm were obtained for ZnO nanoparicles synthesized from Zinc chloride & Zinc nitrate respectively. The Scanning Electron Microscope (SEM) pictures show the morphology of nanoparticles. The FTIR spectrum shows that the characteristic absorption peaks of Zn-O are nearer to 390 cm-1. The sharp characteristic peaks are also observed in FTIR spectrum of ZnO particles synthesized from Zinc Nitrate suggesting the high crystalline nature of ZnO nanoparticles.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML