-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2015; 5(1): 17-21
doi:10.5923/j.materials.20150501.03
Preparation and Characteristics of Mercaptobenzene Functionalized Graphite and Epoxy-Based Hybrid Membranes
Ayesha Kausar
Nanosciences and Catalysis Division, National Centre For Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan
Correspondence to: Ayesha Kausar, Nanosciences and Catalysis Division, National Centre For Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Here we report the preparation and characterization of mercaptobenzene functionalized graphite and bisphenol A ethoxylate diacrylate epoxy-based hybrid membranes (BAEDA/Graphite-MB). A series of gold nanoparticle and mercaptobenzene functionalized graphite and epoxy membranes (BAEDA/Graphite-MB-AuNPs) were also prepared. First a free-standing carbon-based membrane material was prepared using nylon filtering membrane. Afterwards, the composite membrane of Epoxy and functionalized graphite was prepared. The permutation of macroscopic structure was the result of unique morphology and improved thermal conductivity in BAEDA/Graphite-MB-AuNPs membranes. The thermal conductivity of BAEDA/Graphite-MB-AuNPs membranes ranged between 2.1 and 5.7 W/mK. The membrane property such as water permeability was also measured using relevant set-up. Higher water permeability of BAEDA/Graphite-MB-AuNPs membranes was observed due to better nanofiller dispersion.
Keywords: Epoxy, Mercaptobenzene, Graphite, Hybrid membranes, Thermal conductivity
Cite this paper: Ayesha Kausar, Preparation and Characteristics of Mercaptobenzene Functionalized Graphite and Epoxy-Based Hybrid Membranes, American Journal of Materials Science, Vol. 5 No. 1, 2015, pp. 17-21. doi: 10.5923/j.materials.20150501.03.
Article Outline
1. Introduction
- Due to simplicity of manufacturing and noteworthy gain in properties, the epoxy nanocomposites have simulated considerable interest in material science [1]. Epoxy-based materials have range of properties such as increased strength, modulus, fracture toughness, compression, impact, gas barrier properties, wear properties, thermal resistance, and flame retardance [2, 3]. The word ‘epoxy resin’ suggests cured resin/hardener system as well as the epoxy pre-polymer forms. The epoxy group consists of a three-membered ring known as epoxide, oxirane, glycidyl, or ethoxyline. An epoxy group is usually highly strained, so increasing its reactivity. Epoxy resins are talented material for several high performance applications such as aerospace and transportation industry owing to their good adhesive properties, mechanical strength, and low density (1.3g/cm2). Consequently, epoxy composites in the form of fiber/honeycomb structures have been utilized in various aerospace parts and aircraft structures [4]. In this regard, graphite foils with stacked platelets of expanded graphite have been employed in automotive gasketing due to chemical resistivity and better sealability at high temperatures [5, 6]. Graphite is a two dimensional layered material consisting of graphene sheets with planar crystal structure. Graphite is an excellent conductor of heat and electricity and has higher strength and stiffness. It maintains its strength and stability up to 3,600°C temperature and is awfully resistant to chemical attack. Graphite sheets bear oxygen functional groups on their basal planes and edges. Graphite-based thin films have been fabricated via solvent-casting methods [7]. In addition, the morphology and mechanical properties of the resulting thin-film materials have been elucidated. In recent times, the functional graphite-based composites have engrossed plenty of scientific and engineering curiosity due to superior mechanical, electrical, and thermal properties. Even very low quantities of graphite filler may produce remarkable improvement in properties [8-10]. Several methods have been utilized to attain successful reinforcement of functional graphite in composites such as in-situ technique, solution method, and melt blending. Among these methods, solution route is most proficient in the formation of homogeneous colloidal suspensions. Solution method may also provide fine processability and feasibility for large-scale production [11]. Graphite can be functionalized using various routes including chemical exfoliation as well as other modification processes [12]. Nowadays, free-standing graphite paper-like nano-materials have been utilized as chemical filters, shielding materials, conducting barriers, and electronic devices. Two forms of carbon-based paper materials have been frequently commercialize such as carbon-nanotube bucky paper and graphite foils [13, 14]. Lately, graphite/graphene-based paper has been investigated as two-dimensional graphene structure is predictable to considerably expand paper properties compared with nanotube bucky paper and graphite/functional graphite/ graphene foil [15, 16]. Extensive research in the area of graphite papers has depicted enhanced mechanical properties, while lower electrical properties. Consequently, graphite papers necessitate additional chemical/thermal reduction in order to fabricate electrically conducting membranes [17, 18]. Here we report the preparation and characterization of mercaptobenzene functionalized graphite and epoxy-based hybrid membranes. First a free-standing carbon-based membrane material was prepared using nylon filtering membrane. Afterwards, the composite membrane of Epoxy/ mercaptobenzene functionalized graphite was prepared. The permutation of macroscopic flexibility was the result of unique morphology and improved thermal and electrical conductivity.
2. Experimental
2.1. Materials
- Graphite nanopowder (particle size <10µm), bisphenol A ethoxylate diacrylate (BAEDA, average Mn ~ 468), 4-aminothiophenol (97%), AuCl3 (99%), 1, 2-dichlorobenzene (99 %), potassium borohydride (KBH4) and N,N-dimethylformamide (DMF, 99.8%) were purchased from Aldrich.
2.2. Measurement
- Field Emission Scanning Electron Microscopy (FE-SEM) of freeze fractured samples was performed using JSM5910, JEOL Japan. We also measured the thermal conductivity of our samples using a HotDisk Transient Plane Source TPS 2500 S (HotDisk AB). The system uses a nickel wire wound in two intertwined spirals as a sensor. An electrical pulse with a power 0.03–0.5 W in the nickel generates a heat pulse that raises the temperature of the sample [19]. The nickel wire is then used as a sensor to measure the heat dissipation. The HotDisk method requires no special sample preparation. The sensor is simply placed between two pieces of the evaluated materials. All measurements were performed at 23 °C.
2.3. Functionalization of graphite (Graphite-MB)
- In a 250 mL round-bottom flask, 0.5 g graphite was dispersed in 100 mL of 1,2-dichlorobenzene with sonication of 0.5 h. To the above suspension, a solution of 0.5 g of 4-aminothiophenol was added in 10 mL of acetonitrile. Afterwards, 1 mL of isoamyl nitrite was added dropwise. The mixture was further sonicated for 1 h at 60 °C. After cooling, the suspension was filtered. The product was washed with excess of DMF to remove unreacted 4-aminothiophenol and isoamyl nitrite. Finally, the product was dried under vacuum at 70 °C for 6 h [20].
2.4. Preparation of Graphite-MB-AuNPs
- 0.1 g of above functionalized graphite was dispersed in 0.5 mm aqueous solution of AuCl3 with sonication of 1h. Afterwards, aqueous solution of KBH4 was added dropwise to the above suspension. The mixture was stirred vigorously for 1h at room temperature. The product was filtered and dried at 60 °C for 4 h.
2.5. Preparation of BAEDA/Graphite-MB and BAEDA/Graphite-MB-AuNPs Membranes
- Graphite-AuNPs was dispersed in water at different concentrations (1-10 mg/mL) using high intensity probe sonicator to form a stable suspension. Subsequently, Graphite-AuNPs membrane was prepared by filtering the suspension through a nylon filtering membrane with the aid of vacuum. The Graphite-AuNPs membrane was thoroughly washed with deionized water. Finally, the membrane was peeled off from the filter and dried in a vacuum oven. Graphite-AuNPs membrane was soaked into a solution of BAEDA in THF (0.1g/mL) for I h. Afterwards the hybrid membranes were dried at 60 °C for 8 h [21]. The hybrid membranes were marked as BAEDA/Graphite-AuNPs 1-10, where number represents the weight percentage of Graphite-AuNPs in hybrid membrane (Figure 1).
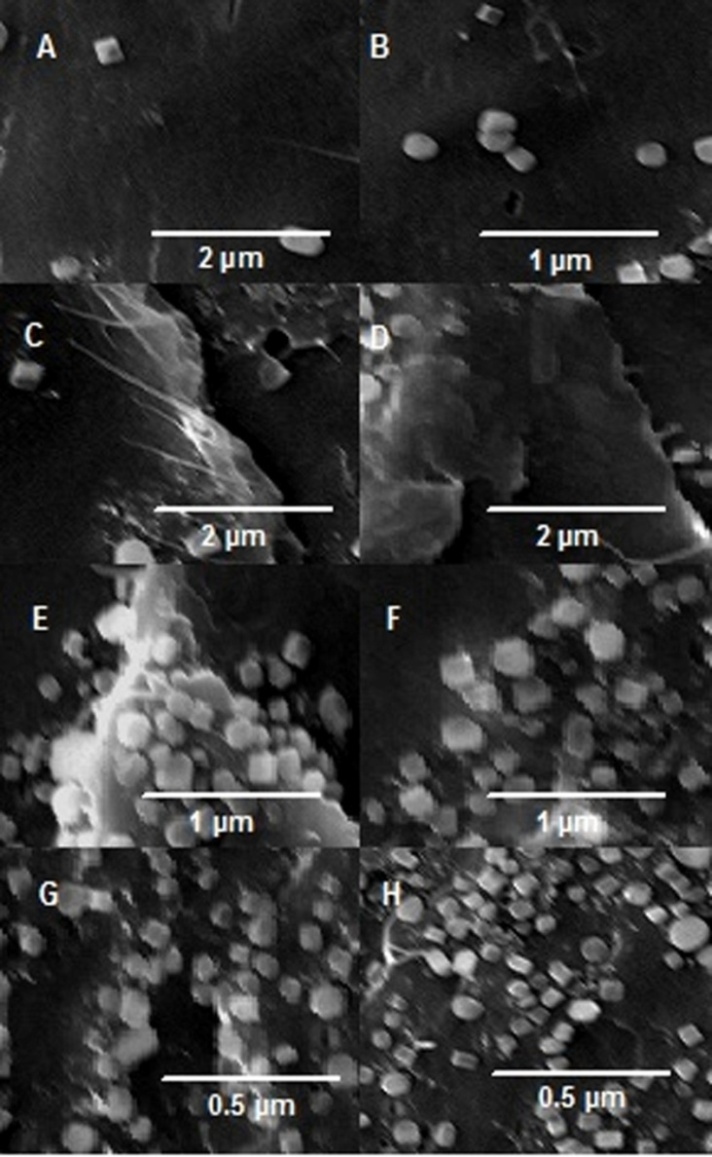
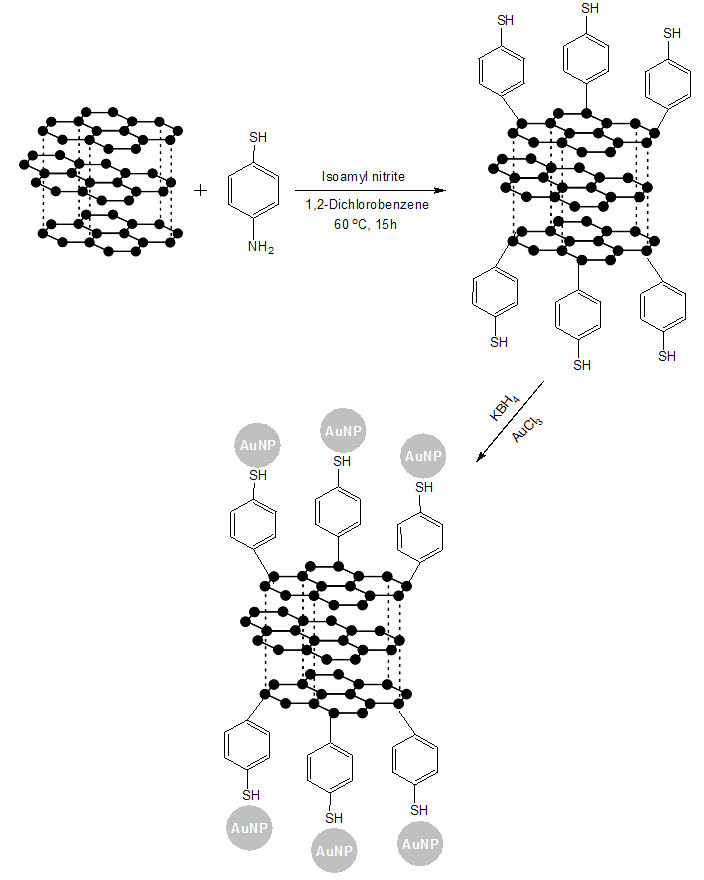 | Figure 1. Chemical modification of graphite with mercaptobenzene moieties and formation of Graphite-MB and Graphite-MB-AuNPs |
3. Results and Discussion
3.1. Morphology of Epoxy/Graphite Membrane
- Figure 2 shows the morphology of BAEDA/Graphite-MB and BAEDA/Graphite-MB-AuNPs composites. FESEM images of mercaptobenzene functionalized graphite and BAEDA epoxy-based hybrid membranes are shown in Figure 2 A-D. It can be seen that the BAEDA/Graphite-MB 1 (Fig. 2 A) and BAEDA/Graphite-MB 2 (Figure 2 B) membranes are smooth and have homogeneous structure. The structure of BAEDA/Graphite-MB 1 membrane was uniform indicating that the Graphite-MB filler was well-dispersed in the suspension. Whereas, the higher filler loading caused dense and layered structure consisting of graphite flakes self-assembled by van der Waals force (Figure 2 C & D). On the other hand, BAEDA/ Graphite-MB-AuNPs membranes (Figure 2 E-H) have shown different morphology. With the addition of AuNPs along with functional graphite, the membranes have revealed the formation of agglomerated particles (Figure 2 E & F). Here, the addition of 1 and 2 wt. % Graphite-MB-AuNPs filler caused coarse dispersion of nanofiller in the epoxy matrix. Further addition of Graphite-MB-AuNPs (5 and 10 wt. %) have depicted fine dispersion of functional graphite along with smaller dispersed particles in BAEDA epoxy matrix (Figure 2 G & H). Interestingly, it can be found that the Graphite-MB-AuNPs was uniformly dispersed in the membrane at high loading and develop good interaction with polymer.
3.2. Permeability of Epoxy/Graphite Membrane
- To estimate the barrier effect of modified graphite filler in epoxy membrane, the water permeability of the produced BAEDA/Graphite-MB-AuNPs was measured. The BAEDA/Graphite-MB-AuNPs membranes were placed inside a filter and sealed. De-ionized water was flowed through the membranes and vacuum was applied to the filter. The surface area and thickness of membranes, water flow rate, and vacuum pressure were recorded to calculate the direction permeability. The direction permeability was calculated by the following equation (Darcy’s law):

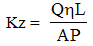 Where Q is flow rate; η is viscosity of water; L is thickness of prepared membranes; and P is the vacuum pressure. Figure 3 shows the water permeability of BAEDA/Graphite- MB-AuNPs membranes. It can be seen that the permeability of BAEDA/Graphite-MB-AuNPs membranes initially decreased with the addition of 1 to 2 wt. % Graphite-MB-AuNPs nanofiller content. The results were closely related with the filler effect, there was an optimum content of Graphite-MB-AuNPs to get the minimum permeability. Later the permeability was improved with the inclusion of 5 wt. % Graphite-MB-AuNPs. From 5 to 10 wt. % nanofiller addition there was continuous increase in permeability depicting the improvement in membrane properties with filler addition. The differences in permeability may be explained on the basis of network formation by the nanoparticles. In fact, the addition of Graphite-MB-AuNPs to epoxy membrane caused two opposite effects on permeability. At lower filler content, the barrier effect was increased due to the formation of resulting agglomerated structure/network. On the one hand, higher filler content may decrease the degree of agglomeration and therefore increase the distance between filler network points, resulting in larger pores. This may increase the permeability of the resultant membrane/network.
Where Q is flow rate; η is viscosity of water; L is thickness of prepared membranes; and P is the vacuum pressure. Figure 3 shows the water permeability of BAEDA/Graphite- MB-AuNPs membranes. It can be seen that the permeability of BAEDA/Graphite-MB-AuNPs membranes initially decreased with the addition of 1 to 2 wt. % Graphite-MB-AuNPs nanofiller content. The results were closely related with the filler effect, there was an optimum content of Graphite-MB-AuNPs to get the minimum permeability. Later the permeability was improved with the inclusion of 5 wt. % Graphite-MB-AuNPs. From 5 to 10 wt. % nanofiller addition there was continuous increase in permeability depicting the improvement in membrane properties with filler addition. The differences in permeability may be explained on the basis of network formation by the nanoparticles. In fact, the addition of Graphite-MB-AuNPs to epoxy membrane caused two opposite effects on permeability. At lower filler content, the barrier effect was increased due to the formation of resulting agglomerated structure/network. On the one hand, higher filler content may decrease the degree of agglomeration and therefore increase the distance between filler network points, resulting in larger pores. This may increase the permeability of the resultant membrane/network.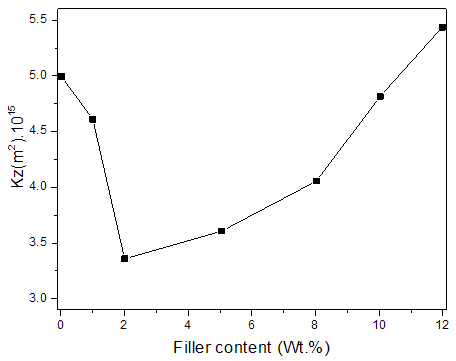 | Figure 3. Water permeability of BAEDA/Graphite-MB-AuNPs membranes |
3.3. Thermal Conductivity
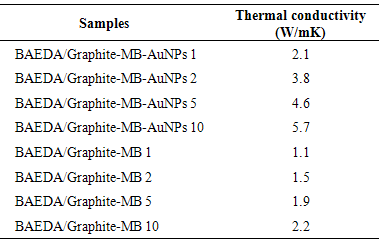
- Figure 4 shows the thermal conductivity effects of Graphite-MB-AuNPs on epoxy membranes. The coefficient of thermal transport was studied by independent Hot-Disk method. For 10 wt. % Graphite-MB-AuNPs-based material, the powder-pressed pellet has thermal conductivity several orders of magnitude higher than 1 wt. % filler loaded sample. Pressed pellets of nanostructures had thermal conductivities 2.1–5.7 W/mK depending on the exact morphology of the composites [22, 23]. On the other hand the BAEDA / Graphite-MB 1-10 series has lower thermal conductivity of 1.1–2.2 W/mK.
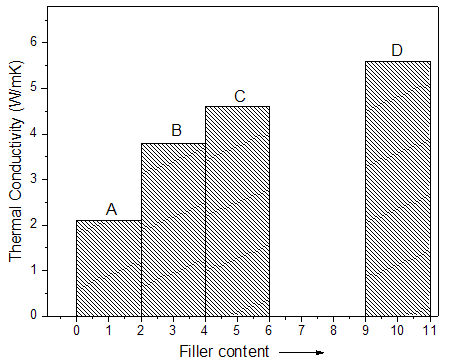 | Figure 4. Thermal conductivity of BAEDA/Graphite-MB-AuNPs membranes |
|
4. Conclusions
- In conclusion, we have investigated the morphology, permeability and thermal conductivity of BAEDA/Graphite-MB and BAEDA/Graphite-MB-AuNPs membranes vs. various nanofiller loading. The better performance of membranes was is due to the homogeneous dispersion and alignment of the modified filler. Better dispersion of filler in composite generally leads to a linear increase of the thermal conductivity with increasing filler content. The thermal conductivity of BAEDA/Graphite-MB- AuNPs loaded with 1 to 10 wt. % filler increased the values accordingly. Similar trend was observed for pellets of BAEDA/Graphite-MB. The permeability of these membranes was first decreased with 1 to 2 wt. % of Graphite-MB-AuNPs nanofiller in the matrix. Whereas, beyond this concentration the properties might increase due to lesser filler aggregation and better dispersion.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML