-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2014; 4(5): 194-201
doi:10.5923/j.materials.20140405.03
Corrosion Inhibition and Adsorption Characteristics of Jatropha curcas Leaves Extract on Aluminium in 1M HCl
Ejikeme P. M.1, 2, Umana S. G.1, Alinnor I. J.3, Onukwuli O. D.4, Menkiti M. C.4
1Department of Pure and Industrial Chemistry, University of Nigeria, Nsukka
2Department of Chemistry, University of Pretoria, Pretoria, South Africa
3Department of Pure and Industrial Chemistry, Federal University of Technology, Owerri-Nigeria
4Department of Chemical Engineering, Nnamdi Azikiwe University, Awka-Nigeria
Correspondence to: Ejikeme P. M., Department of Pure and Industrial Chemistry, University of Nigeria, Nsukka.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Corrosion inhibition of aluminium (AA 1060) in 1M HCl by Jatropha curcas leave extracts was studied using weight loss method at 30℃, 40℃, 50℃ and 60℃. The results obtained indicate that the extracts inhibited the corrosion process in the medium by adsorption and that corrosion rate increased with rise in temperature. Corrosion rate was also found to decrease in the presence of the inhibitor as compared to the blank. Inhibition efficiency was found to increase with increased inhibitor concentration but reduces with increase in temperature to a maximum of 76.49% at 30℃ and 0.5g/L concentration of the leave extract. The change in Gibbs free energy of adsorption was -16.99kJ/mol at 30℃ and decrease with increase in temperature to -13.54kJ/mol at 60℃. The adsorption of the plant extract on aluminium surface was found to obey Temkin, Freundlich and El-Awady adsorption isotherms. Physical adsorption mechanism is proposed for the adsorption of the leaves extract on the aluminium surface.
Keywords: Jatropha Curcas, Heat of Adsorption, Corrosion Inhibition, Physical Adsorption
Cite this paper: Ejikeme P. M., Umana S. G., Alinnor I. J., Onukwuli O. D., Menkiti M. C., Corrosion Inhibition and Adsorption Characteristics of Jatropha curcas Leaves Extract on Aluminium in 1M HCl, American Journal of Materials Science, Vol. 4 No. 5, 2014, pp. 194-201. doi: 10.5923/j.materials.20140405.03.
1. Introduction
- Aluminium, Al is a metallic material that has properties that allow quite different uses and somewhat opposites. Due to its resistance to corrosion in many media and their mechanical properties, reflected in a low density and good mechanical tensile strength, it is a material with excellent characteristics for use in construction and aerospace industry [1]. One of the most important applications of Al and its alloys is found in aluminium-air technology, which was of particular interest for its application to electric vehicle propulsion as well as its low production cost and the existence of a large base for manufacture and distribution [2]. Acid solutions have significant application in industrial processes which includes acid cleaning, descaling and pickling of metallic structures, oil well acidification, electroplating and electro polishing which are usually accompanied by considerable dissolutions of the metal. A useful method of protecting metals like Al and alloys deployed in service in aggressive environments against corrosion is addition of species to the solution in contact with the surface in order to inhibit the corrosion reaction and hence reduce the corrosion rate [3, 4]. Adsorption depends mainly on the charge and nature of the metal surface, electronic characteristics of the metal surface, adsorption of solvent and other ionic species, temperature of corrosion reaction and on the electrochemical potential at solution interface and the type of electrolyte [5]. Originally, inorganic inhibitors such as zinc chromate, polyphosphate and nitrite were used as inhibitors. However, discharge of such material has become unacceptable due to their toxicity and environmental hazards [6]. Accordingly therefore, greater research efforts have been directed towards formulating environmentally acceptable inhibitors. The efficacy of plant extract to act as a successful corrosion inhibitor is its ability to get adsorbed on the surface of the metal [7]. Therefore, the corrosion rate in the presence of the inhibitor may be taken to represent the number of potentially corroding sites that remain after blockage via the plant extract adsorption. It has been observed [8] that the extent and mode of adsorption of corrosion inhibitors on the metal surface depends mainly on certain physico-chemical properties of the molecule such as functional groups, aromaticity, steric factors, electron density at the donor atoms and π orbital character of the donating electrons, and also on the electronic structure of the molecules.Recent awareness emphasizes that plant products containing compounds like tannins, alkaloids, saponnins, essential oils, flavonoids, organic and amino acids are known to exhibit corrosion inhibiting action for steel and aluminium in acidic environment [9-14]. Such organic compounds are known to contain oxygen, nitrogen, phosphorus, and sulphur, in a conjugated system [8] and they inhibit via adsorption of the molecule on the metal surface thereby blocking the active corrosion sites [15]. The extract from plant leaves, roots and seeds have been studied and found to be cheap, environmentally friendly and good corrosion inhibitors. Among the plant leave extract studied include opuntia extract [16], carica papaya and Azadirachta indica [17], Vernonia amygdalina [18], Telfaria occidentalis [19], Phyllanthus amarus [20], fenugreek leaves [21], olive leaves [22], Euphorbia hirta and Dialum guieense [23]. Among the roots studied are ginseng [24], Beet [25], Kopsia singapurensis extract [26] and Murraya koenighii [27] bamboo leaf extract [28]. In the light of the trend in the application of environmentally benign materials as corrosion inhibitors, we report in this study the adsorptive behaviour and evaluation of the corrosion inhibition performance of Jatropha curcas leaves extract. Jatropha curcas is a flowering/ornamental plant. It is a hedge plant and live-fence used to demarcate farms especially in Northern Africa and South Eastern Nigeria. The oil and seeds are used as purgative to cure ring worm, craw-craw, skin infections, rheumatic pains, dropsy and paralysis by rubbing on the affected parts [29]. The leaves have also been shown to effectively act as a sensitizer in Gratzel Cells [30].
2. Experimental Procedure
- Materials: Aluminium sheets of type AA 1060 with 98% purity was purchased at system metals industries limited Calabar, Nigeria. The sheets were mechanically press-cut into coupons of dimension 5cm x 4cm with the thickness of 0.14cm. They were degreased by washing in absolute ethanol and the surface polished with emery paper. The coupons were then re-washed in absolute ethanol, rinsed in acetone to dry and stored in a moisture-free desiccator prior to use. All reagents used for the study were of BDH analytical grade.Plant Extract: The leaves of Jatropha curcas were obtained from Afaha Offiong in Nsit Ibom Local Government Area of Akwa Ibom State, Nigeria. The leaves were sun-dried and ground to powder and 300g of the powdered leaves weighed and soaked in 96% ethanol in a glass column. After 72 hours the extract was ran out of the column and concentrated to paste using the rotatory evaporator. Various masses of the inhibitor, ranging from 0.1g – 0.5g were weighed and dissolved in 1dm3 of 1M HCl.Weight Loss Method: In weight loss method, previously weighed aluminium coupons were immersed in 100ml of 1M HCl solution in an open beaker. In each experiment, the cleaned aluminium coupons was weighed and suspended with the aid of glass rod and hook in a beaker containing 100ml acid solution. The beaker was placed in a water bath maintained at temperatures of 30, 40, 50 and 60℃ respectively. The procedures were conducted with and without various concentrations of the inhibitors. After 8h, the aluminium coupons were withdrawn from the test solution and immersed in 70% nitric acid for 3 minutes at room temperature. They were scrubbed with a bristle brush under running water, dried in acetone and re-weighed. The weight loss was taken as the difference in weight of the coupon before and after immersion determined by a digital weighing balance. The test was conducted in triplicate to guarantee reliability of the result and the mean value of the weight loss was reported.
3. Result and Discussion
- Corrosion Rate and Inhibition Efficiency: Assessment of corrosion rate for aluminium in the different inhibitor/corroding solution was carried out using Eqn. 1 [28].
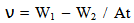 | (1) |
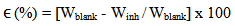 | (2) |
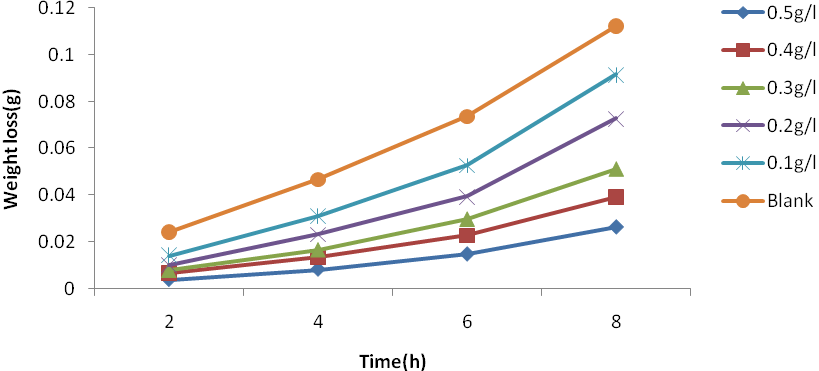 | Figure 1. Plot of weight loss against time for Al corrosion in 1M HCl with JCLE at 30℃ |
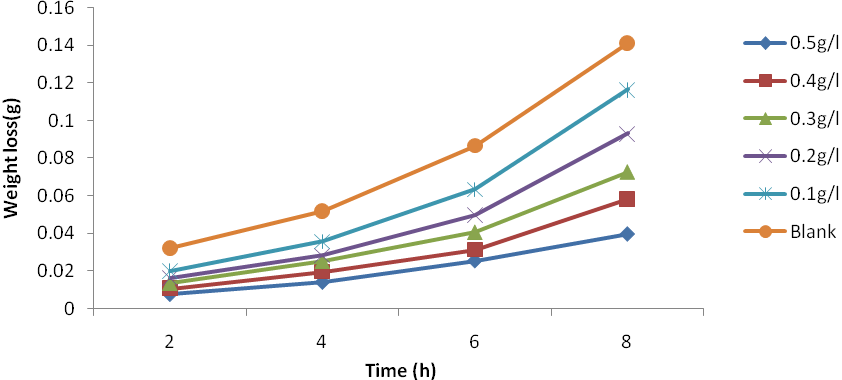 | Figure 2. Plot of weight loss against time for Al corrosion in 1M HCl with JCLE at 40℃ |
 | Figure 3. Plot of weight loss against time for Al corrosion in 1M HCl with JCLE at 50℃ |
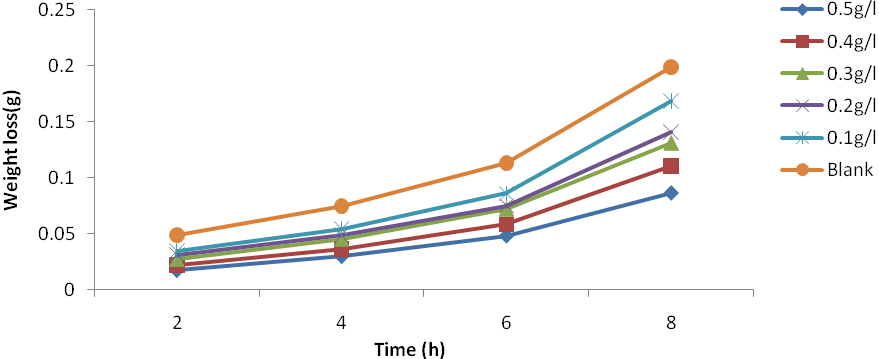 | Figure 4. Plot of weight loss against time for Al corrosion in 1M HCl with JCLE at 60℃ |
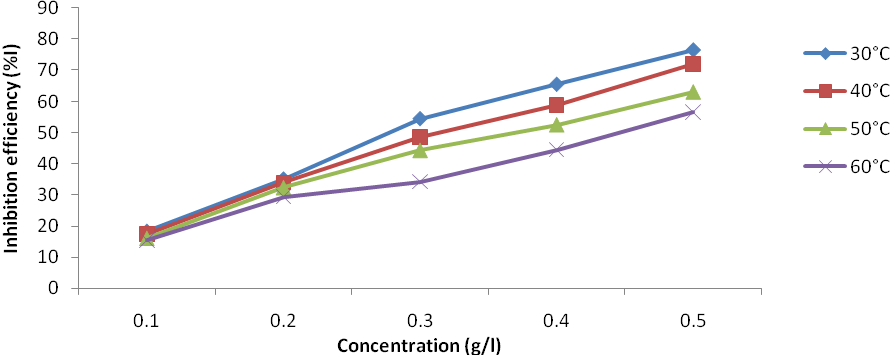 | Figure 5. Plot of inhibition efficiency vs. concentration for Al corrosion in 1M HCl with JCLE |
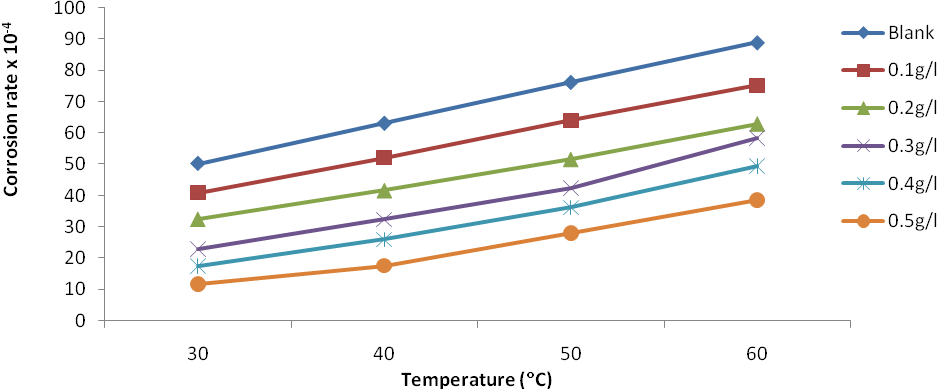 | Figure 6. Plot of corrosion rate against temperature for Al corrosion in 1M HCl with JCLE |
|
 | (3) |
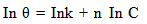 | (4) |
 | (5) |
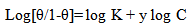 | (6) |
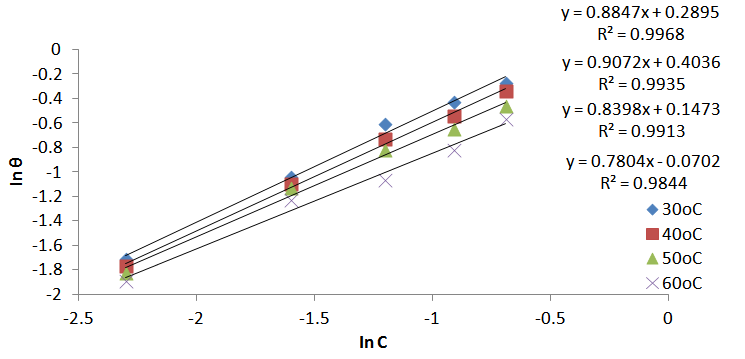 | Figure 7. Freundlich adsorption isotherm for Al corrosion in 1M HCl with JCLE at 30-60℃ |
 | Figure 8. Temkin adsorption isotherm for Al corrosion in 1M HCl with JCLE at 30 - 60℃ |
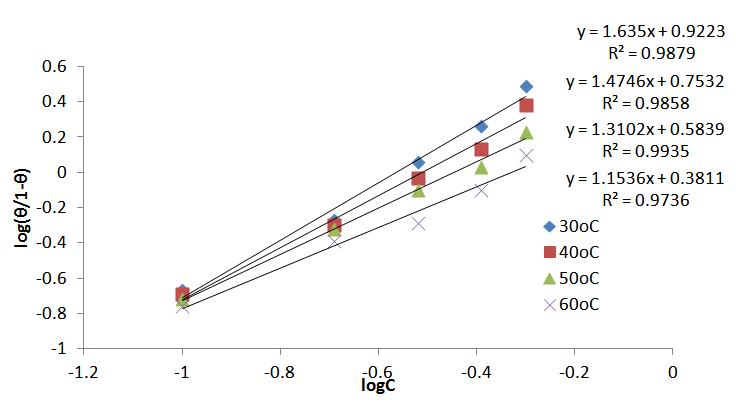 | Figure 9. El-Awady adsorption isotherm for Al corrosion in 1M HCl with JCLE at 30-60℃ |
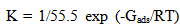 | (6) |
|
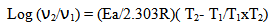 | (7) |
 | (8) |
|
4. Conclusions
- Jatropha curcas leave extract have been found to act as corrosion inhibitor on aluminium in 1M hydrochloric acid solution. The maximum inhibition efficiency of the extract was found to be 76.49% at a concentration of 0.5g/L of the extract. The adsorption of JCLE on the surface of aluminium in 1M HCl followed Freundlich, El-Awady and Temkin adsorption isotherms, in that order. The calculated values of Qads for aluminium in the acid with JCLE were all negative and were of the range of -6.11 to -25.33kJ/mol. A mechanism of physical adsorption is proposed for the process.
References
| [1] | Brito, P.S.D. and Sequeira, C.A.C., 2013, Organic Inhibitors of the Anode Self-Corrosion in Aluminum-Air Batteries, Journal of Fuel Cell Science and Technology, 11(1), 011008. DOI: 10.1115/1.4025534. |
| [2] | Amin M.A., Abd EI-Rehim, S.S., El-Sherbini, E.E.F., Hazzazi, O.A. and Abbas, M.N., 2009, Polyacrylic acid as a corrosion inhibitor for aluminium in weakly alkaline solutions. Part I: Weight loss, polarization, impedance EFM and EDX studies, Corrosion Science, 51, 658-667. |
| [3] | Nnanna, L.A., Onwuagba, B.N., Mejeha, I.M. and Okeoma, K.B., 2010, Inhibition effects of some plant extracts on the acid corrosion of aluminium alloy, African Journal of Pure and Applied Chemistry, 4(1), 11-16. |
| [4] | Rahuma, M.N., Amer, H. and Alfergani, M., 2014, Corrosion inhibition of mild steel in 11% hydrochloric acid solutions by using black pepper (Piper Nigrum), Chemical Science Transactions, 3(2), 764-772. |
| [5] | Oguzie, E.E., 2004, Influence of halide ions on the inhibitive effect of congo red dye on the corrosion of mild steel in sulphuric acid solution, Material Chemistry and Physics 87, 212-217. |
| [6] | Sharmila, A., Prema, A.A. and Sahayaraj, P.A., 2010, Influence of Murraya koenigii (curry leaves) extract on the corrosion inibition of carbon steel in HCl solution, Rasayan Journal of Chemistry, 3(1), 74-81. |
| [7] | Morales-Gil, P., Walczak, M.S., Cottis, R.A., Romero, J.M. and Lindsay, R., 2014, Corrosion inhibitor binding in an acidic medium: Interaction of 2-mercaptobenizmidazole with carbon-steel in hydrochloric acid, Corrosion Science, 85, 109–114. |
| [8] | Mobin M. and Khan, M.A., 2013, Investigation on the Adsorption and Corrosion Inhibition Behavior of Gum Acacia and Synergistic Surfactants Additives on Mild Steel in 0.1 m H2SO4, Journal of Dispersion Science and Technology, DOI: 10.1080/01932691.2012.751031. |
| [9] | Obot, I.B., Obi-Egbedi, N.O. and Umoren, S.A., 2009, Adsorption characteristics and corrosion inhibitive properties of clotrimazole for aluminium corrosion in hydrochloric acid, International Journal of Electrochemical Science, 4, 863-877. |
| [10] | Oguzie, E.E., Okolie B.N., Ogukwe, C.E. and Unaegbu, C., 2006, Corrosion inhibition and adsorption behaviour of bismark brown dye on aluminium in sodium hydroxide solutions, Materials Letter, 60, 3376-3378. |
| [11] | Begum, A.S., Mallika, J. and Gayathri, P., 2010, Corrosion inhibition properties of some 1,3,4-thiadiazolines on mild steel in acidic medium, Journal of Chemistry, 7(1), 117-122. |
| [12] | Jeyaprabha, C., Sathiyanarayanan, S., Phani, K.L.N. and Venkatachari, G., 2005, Influence of poly(aminoquinone) on corrosion inhibition of iron in acid media, Applied Surface Science, 252, 966-975. |
| [13] | Oguzie, E.E., Okolue, B.N., Ebenso, E.E., Onuoha, G.N. and Onuchukwu, A.I., 2004, Evaluation of inhibitory effect of methylene blue dye on the corrosion of aluminium in hydrochloric acid, Material Chemistry and Physics, 87(2/3), 394-401. |
| [14] | Ebenso, E.E. Ekpe, U.J. Umoren, S. Jackson, E. Abiola, O.K. Oforka, N.C. and Martinez, S., 2004, Corrosion Inhibition Studies of some plant extracts on Aluminium in acidic medium, Journal of Corrosion Science and Technology, (1), 96-102. |
| [15] | Ebenso, E.E. and Oguzie, E.E., 2005, Corrosion inhibition of mild steel in acidic media by some organic dyes, Material Letter, 59(17), 2163-2165. |
| [16] | El-Etre, A.Y., 2003, Inhibition of aluminium corrosion using opuntia extract, Corrosion Science, 45(11), 2485-2495. |
| [17] | Ebenso, E.E., Ibok, U.J., Ekpe, U.J., Umoren, S., Jackson, E., Abiola, O.K., Oforka, N.C. and Martinez, S., 2004, Corrosion inhibition studies of plant extracts on aluminium in acidic medium, Transactions of the Society for Advancement of Electrochemical Science and Technology, 39, 117-123. |
| [18] | Odiongenyi, A.D., Odoemelam, S.A. and Eddy, N.O., 2009, Corrosion inhibition and adsorption properties of ethanol extract of Vernonia amygdalina for the corrosion of mild steel in H2SO4, Portugaliae Electrochimica Acta, 27(1), 33-45. |
| [19] | Oguzie, E.E., 2005, Inhibition of acid corrosion in mild steel by telfaria occidentalis extract, Pigment and Resin Technology, 34(6), 321-326. |
| [20] | Okafor, P.C., Ikpi, M.E., Uwah, I.E., Ebenso, E.E., Ekpe, U.J. and Umoren, S.A., 2008, Inhibitory action of phyllanthus amarus extracts on the corrosion of mild steel in acidic media, Corrosion Science, 50, 2310-2317. |
| [21] | Noor, E.A., 2007, Temperature effects on the corrosion inhibition of mild steel in acidic solutions by aqeous extract of fenugreek leaves, International Journal of Electrochemical Science, 2, 996-1017. |
| [22] | El-Etre, A.Y., 2007, Inhibition of acid corrosion of carbon steel using aqeous extract of olive leaves, Journal of Colloid and Interface Science, 314, 578-583. |
| [23] | Umoren, S.A., 2008, Inhibition of aluminium and mild steel corrosion in acidic medium using gum Arabic, Cellulose, 15, 751-761. |
| [24] | Obot, I.B. and Obi-Egbedi, N.O., 2009, Ginseng Root: A new efficient and effective eco-friendly corrosion inhibitor for aluminium alloy of type AA 1060 in hydrochloric acid solution, International Journal of Electrochemical Science, 4, 1277-1288. |
| [25] | Arokia, S.J., Rajendran, S., Ganga, S.V., Amalraj, A.J. and Naranyansamy, B., 2009, Corrosion inhibition of beet root extract, Portugaliae Electrochemica Acta, 27(1), 1-11. |
| [26] | Raja P. B., Rahim, A.A., Osman H. and Awang, K., 2010, Inhibitory effect of Kopsia singapurensis extract on the corrosion behaviour of mild steel in acid media, Acta Physica Chimica Sinica, 26, 1-9. |
| [27] | Sharmila A., Prema, A. and Sahayaraj, A., 2010, Influence of Murraya koenighii extract on the corrosion inhibition of carbon steel in HCl solution, Rasayan Journal of Chemical Science, 3(1), 74-81. |
| [28] | Li X., Deng S., Fu, H., 2012, Inhibition of the corrosion of steel in HCl, H2SO4 solutions by bamboo leaf extract, Corrosion Science, 62, 163–175. |
| [29] | Etukudoh, I. Ethnobotany: Conventional and traditional use of plant, Vol. 1. Uyo Nigeria: The Verdict Press 2003. |
| [30] | Nwanya, A.C., Ugwuoke, P.E., Ejikeme, P.M., Oparaku, O.U. and Ezema, F.I., 2012, Jathropha curcas and Citrus aurantium leaves dye extract for use in dye sensitized solar cell with TiO2 films, International Journal of Electrochemical Science, 7(11), 11219 - 11235. |
| [31] | Li, W. Zhao, X. Liu, F. and Hou, B., 2008, Investigation on inhibition behavior of S-triazole–triazole derivatives in acidic solution, Corrosion Science, 50(11), 3261-3266. |
| [32] | Umoren S.A., Obot I.B., Ebenso, E.E., Obi-Egbedi, N., 2008, Studies on the inhibitive effect of exudate gum from Dacroydes edulis on the acid corrosion of aluminium, Portugaliae Electrochimica Acta, 26, 199-209. |
| [33] | Bouklah, M. and Hammouti, B., 2006, Thermodynamic characterisation of steel corrosion for the corrosion inhibition of steel in sulphuris acid solutions by Artemisia, Portugaliae Electrochimica Acta, 24, 457-468. |
| [34] | Umoren, S.A., Obot, I.B., Ebenso, E.E., Okafor, P.C., Ogbobe, O. and Oguzie, E.E., 2006, Gum arabic as a potential corrosion inhibitor for aluminium in alkaline medium and its adsorption characteristics, Anti-Corrosion Methods and Materials, 53(5), 277-282. |
| [35] | Herrag, L, Chetouani, A, Elkadiri, S, Hammouti, B and Aouniti, A., 2008, Pyrazole Derivatives as Corrosion Inhibitors for Steel in Hydrochloric Acid, Portugaliae Electrochimica Acta, 26(2), 211-220. |
| [36] | Oguzie, E.E., 2006, Adsorption and corrosion inhibitive properties of Azadirachta indica in acid solutions, Pigment and Resin Technology, 35(6), 334-340. |
| [37] | Bouyanzer, A., Hammouti, B., and Majidi, L., 2006, Penny royal oil from menthe pulegium as corrosion inhibitor for steel in IM HCl, Material Letters, 60, 2840-2843. |
| [38] | Oguzie E.E., 2007, Corrosion inhibition of aluminium in acidic and alkaline media by Sansevieria trifasciata extract, Corrosion Science, 49, 1527-1539. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML