-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2014; 4(4): 178-183
doi:10.5923/j.materials.20140404.04
Acidic Corrosion Inhibition of Piper guineense Seed Extract on Al Alloy
Nwosu O. F.1, Osarolube E.2, Nnanna L. A.1, Akoma C. S.1, Chigbu T.1
1Department of Physics/Electronics, Abia State Polytechnic, Aba, Abia State
2Physics Department, University of Port Harcourt, Choba, Rivers State
Correspondence to: Nwosu O. F., Department of Physics/Electronics, Abia State Polytechnic, Aba, Abia State.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Many attempts are being made to curb the menace of corrosion. Recently, green inhibitors are widely considered due to their comparative advantage over other means of corrosion control and prevention. In this study, gravimetric technique was employed to study the inhibitive behaviour of Piper guineense seed extract on Al alloy in acidic environment. The investigation showed optimal inhibition efficiency of about 95.34%. Piper guineense seed extract was adsorbed on the Al alloy surface in accordance with Langmuir adsorption isotherm models. The negative adsorption energy 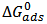 obtained inferred that the adsorption rates were spontaneous and the interaction between the inhibitive molecules was found to be repulsive.
obtained inferred that the adsorption rates were spontaneous and the interaction between the inhibitive molecules was found to be repulsive.
Keywords: Green inhibitor, Corrosion inhibition, Piper guineense, Adsorption Isotherm
Cite this paper: Nwosu O. F., Osarolube E., Nnanna L. A., Akoma C. S., Chigbu T., Acidic Corrosion Inhibition of Piper guineense Seed Extract on Al Alloy, American Journal of Materials Science, Vol. 4 No. 4, 2014, pp. 178-183. doi: 10.5923/j.materials.20140404.04.
1. Introduction
- Aluminium alloys are widely employed in engineering and in architectural construction because of a favorable combination of properties such as strength, lightness, corrosion resistance, electrical and thermal conductivity and attractive appearance [1, 2]. The site where Al and its alloy are used exposes them to corrosion attack. For example, acidic rain as pointed out by Owate et al in 1999 [3] was found to affect the electrical properties of household appliances. Furthermore, kele et al in 2008 [4] and Nwosu et al in 2013 [5] differently reported that acid solutions are often used in industries for cleaning, descaling and pickling of metallic structures, processes which are normally accompanied by considerable dissolution of the metal. Also, excessive corrosion attack is known to occur on metals deployed in service in aggressive environments [6].Among several techniques used in corrosion control/prevention (such as lubrication, painting, cathodic and anodic protection, and electro-painting, material selection, etc), [7] made known that the use of green inhibitors has generated a lot of attention. Some of the advantages of green inhibitors over the rest could be attributed to the following: green inhibitors are cheap, eco-friendly, non-toxic, biodegradable, and they do not contain heavy metals. However, inhibitors are (organic and/or inorganic) chemical compounds that are usually used in small concentrations whenever a metal is in contact with an aggressive medium. The presence of such compounds retards the corrosion process and keeps its rate to a minimum and thus prevents economic losses due to metallic corrosion [8].Recent awareness emphasizes that plant products containing tannins, alkaloids, Saponnins, essential oils, flavonoids, organic and amino acids are known to exhibit corrosion inhibiting action [9-13]. One of the mechanisms employed by the plant molecules is to adhere to the metal surface thereby restricting the aggressive liquid from touching the metal surface. Given the growing trend, this research aims at finding the inhibitive action of Piper guineense seed extract in 1.0 M HCl acidic medium. Piper guineense is a spice plant from the family, Piperaceae and from genus piper commonly called Ashanti. The seeds, leaves and sometimes the stems are used in preparing local dish. It imparts “heat” and a spicy pungent aroma to food. The medicinal properties of Piper guineense exert bacteriostatic and bacteriocidal effects on some bacteria. They are also used for the treatment of cough, bronchitis, intestinal disease and rheumatism. It is a climbing plant that can grow up to 20 m in length. The seeds are smooth and are prolate-elliptically shaped. The presence of the following Phytochemicals are found in Piper guineense: alkaloids, flavonoids, phenols, cardiac and glycosides [14].
2. Experimental Procedure
- Material Preparation: The Al alloy procured from First Aluminium PLC, Port-Harcourt was mechanically cut into 40 mm x 20 mm coupons. The % weight compositions of the Al alloy are: Al (98.473), Si (0.456), Fe (0.760), Cu (0.069), Mn (0.116), Mg (0.020), Zn (0.053), Ti (0.016), Cr (0.005), Ni (0.004), V (0.007), Pb (0.021). A uniform hole of diameter (2 mm) was drilled to facilitate suspension of the coupon in the test solution. The coupons were mechanically cleaned. The cleaning was followed by polishing with emery paper (of different grates: 600, 800, 1000 and 1200) of fine quality to expose shining polished surface. To remove any oil and organic impurities, coupons were washed with detergent, degreased in ethanol and in acetone and finally rinsed with de-ionized water, dried in air and then stored in a desiccator. Accurate weight of the coupons was taken using FA2104A analytical electronic digital weighing balance and recorded as initial weight, Wi.Preparation of Piper guineense Seed Extract: The seeds harvested form Uzuakoli forest (Lat: 5°37'39.29״; Long: 7°33'18.94'') in Abia state, Nigeria were washed in running water, sun dried. Then the seeds were ground into powder. The extraction was carried out in a reflux set-up [15, 16, 17, 18, 4] for 3 hrs at a constant temperature of 323 ± 2 K using 13.3 g of ground stock 400 ml of 1.0 M HCl solution. The resulting solution was allowed to cool to room temperature, filtered and stored. Different concentrations (0.0, 0.1, 0.2, 0.3, 0.4 and 0.5 g/L) of the Piper guineense was prepared in 1.0 M HCl. Gravimetric Experiment: The pre-weighed specimen (Al alloy) were labeled and immersed in 250 mL beaker containing 240 mL test solutions of the prepared inhibitors at room temperature (303 K). Similar procedure had been reported by [19-21]. The set up were exposed for a maximum period of eight hours and each specimen was retrieved from the different test media after an interval of one hour. After immersion, the sample coupon was cleaned, washed in distilled water several time, degreased in ethanol and dried in acetone. Further drying of the coupon was done by means of a hand dryer and then reweighed on cooling in order to determine the weight loss of the coupon.
3. Results and Discussion
- The weight loss of aluminium alloy was determined at various intervals in the presence and absence of different concentrations of the investigated spice extract. The obtained variations of weight loss with exposure time are presented in figure 1. The degree of metal dissolution is dependent on the surface area of the metal and the time of exposure known as the corrosion rate is expressed in eq. 1. [22].
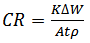 | (1) |
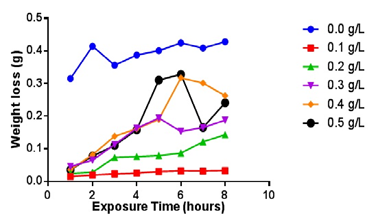 | Figure 1. Varaition of Weight loss (g) with time (hours) of Al alloy in different concentration of Piper guineense extract in 1.0M HCL solution |
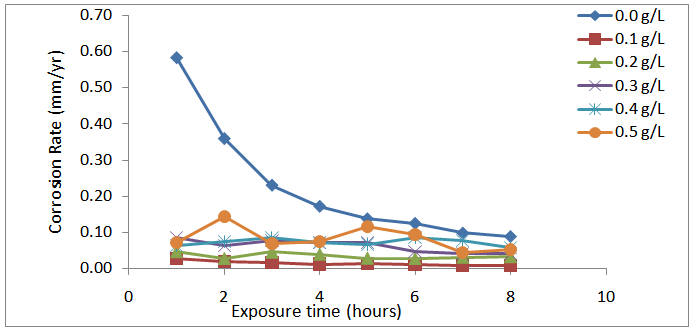 | Figure 2. Variation of Corrosion rate (mm/yr) with time (hours) of Al alloy in 1.0 M HCl containing different concentrations of Piper guineense seed extract |
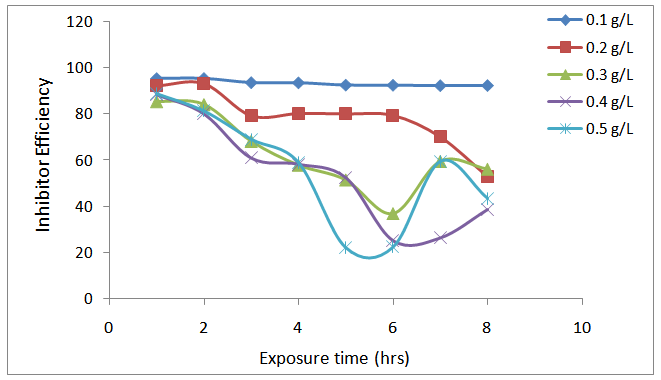 | Figure 3. Variation of inhibition efficiency with inhibitor concentraion of Piper guineense seed extract on Al alloy in 1.0 M HCl solution |
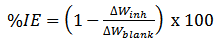 | (2) |
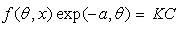 | (3) |
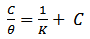 | (4) |
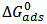 is related to K (in eq. 5) [33]. The plot of C/θ against C (figure 4) reveals that the experimental date fit to the theoretical. The curve yields a straight line with slope = 1.31 and correlation coefficient (R2) = 0.998. The K = 30.3 was obtained from the intercept of figure 4.
is related to K (in eq. 5) [33]. The plot of C/θ against C (figure 4) reveals that the experimental date fit to the theoretical. The curve yields a straight line with slope = 1.31 and correlation coefficient (R2) = 0.998. The K = 30.3 was obtained from the intercept of figure 4. 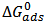 = -18.7 kJ/mol was calculated using eq. 7. The plot obeys Langmuir adsorption isotherm as the plot has linearity and good correlation coefficient. The R2 value is very close to unity, indicating strong adherence to Langmuir adsorption isotherm [34].
= -18.7 kJ/mol was calculated using eq. 7. The plot obeys Langmuir adsorption isotherm as the plot has linearity and good correlation coefficient. The R2 value is very close to unity, indicating strong adherence to Langmuir adsorption isotherm [34].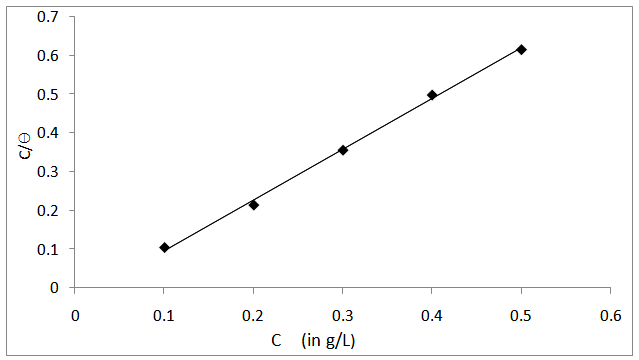 | Figure 4. Langmuir isotherm of Al alloy in 1.0 M HCl containing Piper guineense seed extract |
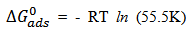 | (5) |
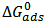 is below 40 kJ/mol, it corroborates that the adsorption process is Physisorption. The negative value of
is below 40 kJ/mol, it corroborates that the adsorption process is Physisorption. The negative value of 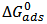 indicated adsorption process of the inhibitor on the Al alloy surface is spontaneous. The interactions involved in this mechanism are more or less weak electrostatic interactions between metal atoms and adsorbate species [19]. Interestingly, the adsorption energies involved in the research have the same range of energy values as the van der Waals bond energies [35].Temkin adsorption model expressed in eq. 6 [16, 17] and its plot in figure 5 reveals that the R2 = 0.913, slope (S = -1.121). From the plot, the molecule interaction parameter f = - 0.892.
indicated adsorption process of the inhibitor on the Al alloy surface is spontaneous. The interactions involved in this mechanism are more or less weak electrostatic interactions between metal atoms and adsorbate species [19]. Interestingly, the adsorption energies involved in the research have the same range of energy values as the van der Waals bond energies [35].Temkin adsorption model expressed in eq. 6 [16, 17] and its plot in figure 5 reveals that the R2 = 0.913, slope (S = -1.121). From the plot, the molecule interaction parameter f = - 0.892. 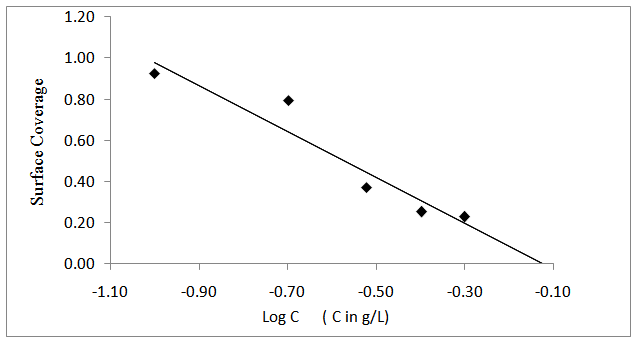 | Figure 5. Temkin Isotherm of Al alloy in 1.0 M HCl containing Piper guineense Seed Extract |
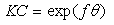 | (6) |
4. Conclusions
- Piper guineense seed extract is an effective corrosion inhibitor for Al alloy in 1.0 M HCl. The inhibitor functions by being adsorbed on Al alloy surface. The mechanism of adsorption of the inhibitor on the metal surface is by physical adsorption. The experimental data fit the Langmuir adsorption isotherm. The adsorption Gibb’s free energy -18.7 kJ/mol confirms that the acidic corrosion inhibition was spontaneous. Temkin adsorption model satisfied that the there exist lateral repulsion of the inhibitor molecules on the metal surface.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML