-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2014; 4(3): 119-126
doi:10.5923/j.materials.20140403.01
Role of Mixed Valence Effect and Orbital Hybridization on Molar Volume of Heavy Metal Glass for Ionic Conduction Pathways Augmentation
F. A. Moustafa 1, M. Abdel-Baki 1, A. M. Fayad 1, Fouad El-Diasty 2
1Glass Department, National Research Centre, Dokki 12311 Giza, Egypt
2Physics department, Faculty of Science, Ain Shams University, Abbasia, 11566 Cairo, Egypt
Correspondence to: Fouad El-Diasty , Physics department, Faculty of Science, Ain Shams University, Abbasia, 11566 Cairo, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Ionic conductivity of glasses can be engineered by understanding the chemical bonding properties and their effect especially on the glass molar volume. Thus, a series of heavy lead borate glass of the composition 0.25B2O3–0.75PbO is prepared by the melt quenching technique. The mixed valence effect and orbital hybridization of 3d-transition metal oxide and 4p-chalcogen combination are used through the co-substitution of Cr2O3 and SeO2 by B2O3 to open up the glass network and to increase the glass molar volume. It provides weaker anionic tetrahedral 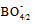 unit linkages instead of strong oxygen linkages of triagonal
unit linkages instead of strong oxygen linkages of triagonal 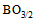 units. Moreover, a gradual conversion of chromium ions from Cr6+ state to Cr3+ state exists where the Cr3+ ion is acting as a modifier to break up the glass local symmetry introducing additional coordinated defects. Among the different negative sites, the existence of nonbridging oxygen bonds and orbital hybridization or pπ-dπ overlaps the molar volume increases and the glass network becomes more open. Thus, it would provide wide pathways for ions like Li+ to be more easily mobile in the glass matrix. Chemical bond approach is used to explain the obtained results.
units. Moreover, a gradual conversion of chromium ions from Cr6+ state to Cr3+ state exists where the Cr3+ ion is acting as a modifier to break up the glass local symmetry introducing additional coordinated defects. Among the different negative sites, the existence of nonbridging oxygen bonds and orbital hybridization or pπ-dπ overlaps the molar volume increases and the glass network becomes more open. Thus, it would provide wide pathways for ions like Li+ to be more easily mobile in the glass matrix. Chemical bond approach is used to explain the obtained results.
Keywords: Glass, Molar volume, p- and d-block elements, Orbital hybridization, Ionic conductivity, Nonbridging oxygen, Bond density
Cite this paper: F. A. Moustafa , M. Abdel-Baki , A. M. Fayad , Fouad El-Diasty , Role of Mixed Valence Effect and Orbital Hybridization on Molar Volume of Heavy Metal Glass for Ionic Conduction Pathways Augmentation, American Journal of Materials Science, Vol. 4 No. 3, 2014, pp. 119-126. doi: 10.5923/j.materials.20140403.01.
Article Outline
1. Introduction
- Metal oxides display physical properties ranging from piezoelectricity to superconductivity, from negative thermal expansion to ionic conductivity and transparent conductors. They are used in computers, Li-ion batteries, fluorescent lights, cellular phones, and fuel cells. Many well-designed properties of the glass are determined by the elements with mixed valences (in which an element has two or more different valences while forming a compound) in the structure unit [1]. High-temperature superconductor [2] was a milestone in the study of metal-oxides and is an effective example for the mixed valence chemistry. Mixed valence effect is required for glasses to stimulate electronic, structural, and chemical progress leading to specific functionality. The valence states of metal cations in glass can be governed chemically using orbital hybridization.Borate glasses are composed of structural groupings as boroxol, tetraborate, diborate, which are linked by bridging oxygens, where the glass consists of microdomains of boroxol rings [3]. Near the glass-transition temperature the boroxol rings break up leading to a more open structure which affects strongly the glass behavior as a host material. In lead borate glass the extreme difference between the masses of the lead and the boron atom are favorable for spectroscopic investigations. This is because increasing the heavy metal oxide content causes an increase in the glass thermal stability. Furthermore, glasses containing cations (such as Pb2+, As3+, or Bi3+) with an outer electron shell of 18 + 2 electrons should have higher polarizabilities and lower melting temperatures [4]. In the same context, lead monoxide possesses interesting semiconducting and photo conducting properties with potential applications in imaging devices, electro photography and laser technology.Selenium has empty d-orbitals which can form four or six bonds by unpairing electrons. Overlap of a p-orbital on the oxygen with a d-orbital on Se gives a pπ-dπ interaction [5]. To obtain effective pπ-dπ overlap, the size of the d-orbital must be similar the size of the p-orbital. Since Se has a covalent radius of 1.14 Åwhile for Cr it is 1.17 Å, therefore, an overlap between them might be highly credible. Upon the glass structure, a strong overlap possibly arise providing 2p-3d orbital hybridization. Such orbital hybridization manifests itself as an increase in glass optical polarizability throughout spin-orbit coupling mechanism [6]. Selenium oxide possesses two kinds of structures as selenite and selenate around Se atom. Selenite (SeO32-) acts as a modifier in the glass network with three coordinated oxygen and one lone pair of electrons, whereas the selenate (SeO42-) with four coordinated oxygen occupies tetrahedral sites in the glass network and acts as a network former [7, 8].The role of an ion in glass environment is largely determined by its ionic radius a, and its effective electrostatic field strength eZ/a2 where eZ is the ionic charge. According to stereochemical reasoning, coordination number four is suggested for small ions to be incorporated in interatomic space between four closed-packed oxygens. Octahedron of close-packed oxygens-coordination number 6-or even more is required for larger ions. Transition metals with valences of +3 or +4 such as Cr, which having a lower electronegativity, with oxides have a higher electronegativity such as SeO2 are required to design the glass optoelectronic structure such as the glass band gap [9-11].Existing of chalcogenide elements in glass network provides a number of amorphous semiconductor-like properties [12, 13]. For instance, increasing the relative atomic mass of the chalcogen or its proportion in the glass reduces its average bond strength [14]. Elements such as Se have electronic structure s2p4 tend to gain two electrons forming M2- ions, or to share two electrons and thus forming two covalent bonds. Many well-designed properties of the glass are obtained by using elements with mixed valences in the structure network [1]. In the present work, 3d-4p orbital replacement of B2O3 by a combination of Cr2O3 andSeO2 is used to engineer the molar volume of a heavy metal borate glass. The chemical bond approach is used to analyze and to explain the role of mixed valence effect on the glass molar volume to enhance the expected glass ionic conductivity.
2. Experimental Details
2.1. Glass Preparation
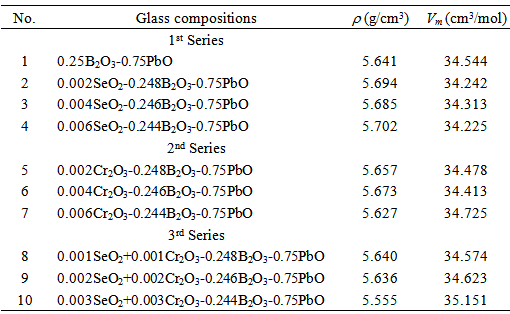
- Three series of lead borate glasses of composition xCr2O3-(0.25-x) B2O3–0.75PbO, xSeO2-(0.25-x)B2O3– 0.75PbOand x(Cr2O3-SeO2)-(0.25-x)B2O3–0.75PbO are prepared, Table 1, where x is the oxide molar fraction. The used materials were of chemically pure grade, in the form of H3BO3, Cr2O3, SeO2 and Pb3O4. The amount of the glass batch was 50 g melt-1. The glass was prepared by melt quenching technique using porcelain crucibles in an electric furnace. The temperature of melting was 1100C, whereas the duration of melting was one hour after the last traces of batches were disappeared. To avoid the presence of bubbles in the glass melt, the melt was continuing stirred during the glass preparation. Then the melt was poured onto stainless steel mould and annealed at around 350oC to remove the thermal strains. Optical slabs were prepared by grinding and polishing of the prepared samples with paraffin oil and minimum amount of water. The thickness of the glass slabs was about 3 mm. Polishing was completed with stannic oxide and paraffin to reach a minimum surface roughness which was tested by an interferometric method. The homogeneity of the glasses was examined using two crossed polarizers.
2.2. Measurement of Glass Density
- The hydrostatic density of the glasses, ρ, at room temperature was measured by the Archimedes principle using a sensitive microbalance. The error in measuring the glass density was 0.001 g/cm3.
3. Results and Discussion
3.1. Density and Molar Volume
- The molar volume, Vm, of the glass was calculated using the expression:
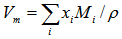 | (1) |
|
3.2. Intermolecular Separation and Bond Density
- Replacement of Cr2O3 or/and SeO2 into the glass network instead of B2O3 makes effective changes for the density and molar volume of the base glass sample (i.e., sample 1). Since the molar masses of Cr2O3 and SeO2 are 99.99 and 110.96 g/mol, respectively, while for B2O3 is 69.622 g/mol, so the replacement of SeO2 by B2O3 explains such increase in the glass density of the base sample with increasing SeO2 content as in samples 5, 6 and 7 (second series). The density and molar volume show opposite behavior (sample 4), where with the same SeO2 amount (x = 0.006) the glass density increased by 1.06% and the molar volume decreased by 1.01%. The behavior of glass density reveals that, with the increase in SeO2 contenta partial reversible transformation occurs for a part of selenite groups (SeO32-) into the more densselenate (SeO42-) with its four coordinated oxygen occupying tetrahedral sites in the glass network. In the same context, the fluctuation in the glass density which is seen with samples1, 2, 3, and 4 (first series) due to replacement of Cr2O3 by B2O3 may beat tributed to the conversion of chromium ions from Cr6+ state to Cr3+ state and vise verse. Sample 7 with Cr2O3 molar fraction 0.006 has the lowest density, second series. It means the conversion of chromium ions from Cr6+ state into Cr3+ state which has the longest ionic radius (0.65 Å) in compare with Cr6+ that has ionic radius 0.36Å. Also the existing of Cr3+ ions acts as modifier which may create an inconsistency in the number of nonbridging oxygen bonds NBO relative to bridging oxygen bonds (BO). Glass samples 8, 9, and 10 (third series), which containa variable equal share of mole fraction, x, for bothCr2O3and SeO2, show a profound decrease in the glass density compared to the base glass sample. Such unexpected density and molar volume behaviors may be attributed to the creation of an excess of nonbridging oxygen bonds which open up the structure of the glass networks.Analyzing the glass molar volume is more informative by correlate it with the role of SeO2 and Cr2O3 hybridization on the number of bonds per unit volume (nb). The number of bonds per unit volume of the glass is calculated using the relationship [15, 16]:
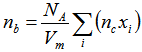 | (2) |
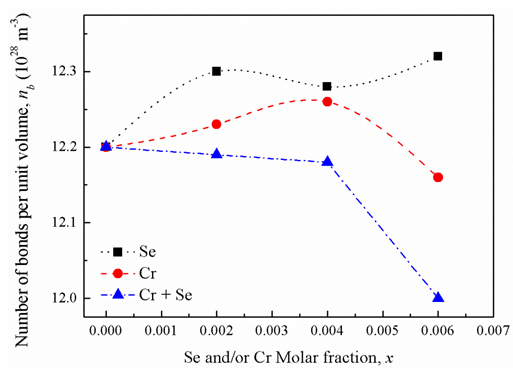 | Figure 1. Number of bonds per unit volume versus oxide molar fractions |
3.3. Oxygen Molar and Packing Densities
- According to chemical bond approach and for further explanation the obtained glass molar volume and density behavior, many additional parameters would also be calculated which are; the volume of glass in which 1 mole of oxygen is contained (the molar volume of oxygen, Vo) and oxygen packing density (OPD). The molar volume of oxygen is given by [17]:
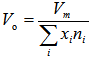 | (3) |
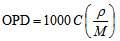 | (4) |
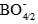 units, which leads to a variation in the ratio between triagonal
units, which leads to a variation in the ratio between triagonal 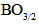 units and the anionic tetrahedral
units and the anionic tetrahedral 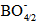 units of the glass network [18, 19].
units of the glass network [18, 19].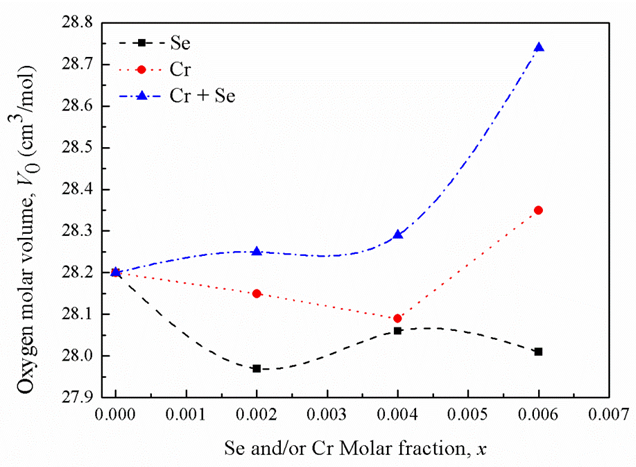 | Figure 2. Oxygen molar volume versus oxide molar fractions |
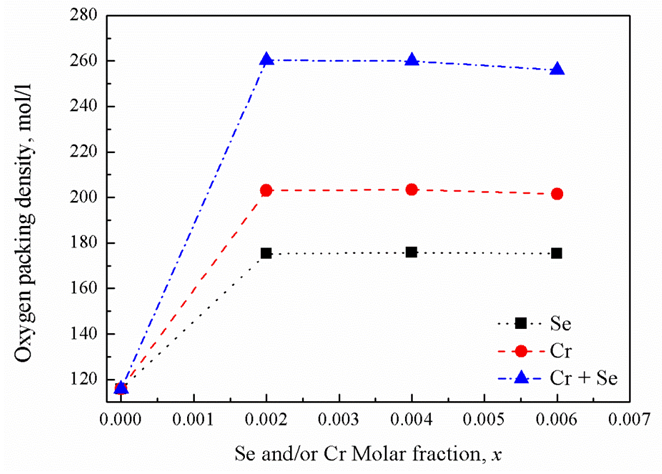 | Figure 3. Oxygen packing density versus oxide molar fractions |
3.4. Boron Molar Volume and Glass Network Free Space
- The average boron-boron separation dB-B is calculated to give more insight on the modification of the glass network due to the presence of SeO2 and Cr2O3. The boron atoms are the central atoms with negatively charged tetrahedral
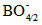 units. Thus the boronmolar volume
units. Thus the boronmolar volume 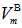 which corresponds to the volume that contains one mole of boron atoms within the specific glass structure is fined as [20]:
which corresponds to the volume that contains one mole of boron atoms within the specific glass structure is fined as [20]: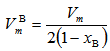 | (5) |
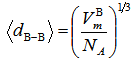 | (6) |
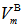 depends on cation species, therefore, the calculated values of average boron-boron separationdB-B are increased with the increase in the SeO2 and Cr2O3 contents from 0.337 nm (sample 1) to 0.344 nm for sample 10. Thus, the incorporation of SeO2 and Cr2O3 on expense of B2O3 leads to a substantial expansion of the glass structural network which confirms the obtained density and molar volume values. Furthermore, the calculated values of boronmolar volume
depends on cation species, therefore, the calculated values of average boron-boron separationdB-B are increased with the increase in the SeO2 and Cr2O3 contents from 0.337 nm (sample 1) to 0.344 nm for sample 10. Thus, the incorporation of SeO2 and Cr2O3 on expense of B2O3 leads to a substantial expansion of the glass structural network which confirms the obtained density and molar volume values. Furthermore, the calculated values of boronmolar volume 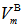 illustrate that with increasing both SeO2 and Cr2O3 contents together on the expense of boron oxide, the
illustrate that with increasing both SeO2 and Cr2O3 contents together on the expense of boron oxide, the 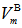 is increased from 23.029 (sample 1) to 23.248 cm3/mol, respectively, as in case of sample 10. Since
is increased from 23.029 (sample 1) to 23.248 cm3/mol, respectively, as in case of sample 10. Since 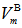 depends on cation species, therefore, with the increase in the SeO2 and Cr2O3 contents, an increase in the average boron-boron separation may be obtained. Thus, the incorporation of SeO2 and Cr2O3 on expense of B2O3 leads to a substantial non densification of the glass structural network which approves the obtained density and molar volume values. The increase in the bond length or inter-atomic spacing between the atoms may be also attributed to a decrease in the stretching force constants of the bonds inside the glass network.Additionally, the decrease in the number of bonds per unit volume, nb, can be investigated by the determination of interatomic distance between Se and Cr atoms. For a glass has an oxide component, i, of xiAbOc, where b and c are the valence of both the anion O and cation A, respectively, then the concentrations of the ions A and O are given by [20]:
depends on cation species, therefore, with the increase in the SeO2 and Cr2O3 contents, an increase in the average boron-boron separation may be obtained. Thus, the incorporation of SeO2 and Cr2O3 on expense of B2O3 leads to a substantial non densification of the glass structural network which approves the obtained density and molar volume values. The increase in the bond length or inter-atomic spacing between the atoms may be also attributed to a decrease in the stretching force constants of the bonds inside the glass network.Additionally, the decrease in the number of bonds per unit volume, nb, can be investigated by the determination of interatomic distance between Se and Cr atoms. For a glass has an oxide component, i, of xiAbOc, where b and c are the valence of both the anion O and cation A, respectively, then the concentrations of the ions A and O are given by [20]: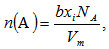 | (7) |
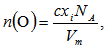 | (8) |
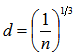 | (9) |
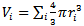 where ri is the radius of the ion i and the summation is taken over all the ions present in one mole of the glass. Then the free space, Vf, in one mole of the glass [20] which is not occupied by the ions is equal to
where ri is the radius of the ion i and the summation is taken over all the ions present in one mole of the glass. Then the free space, Vf, in one mole of the glass [20] which is not occupied by the ions is equal to 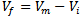 . As shown in Fig. 4, increasing the mole fraction contents of SeO2 and Cr2O3 together up to 0.006 (sample 10) increases the free space per mole from 21.14 (sample 1) to 21.75 cm3/mole, respectively. So the conjunct introduction of SeO2 and Cr2O3 enhances the 2p electron density of the unshared oxygen ion which in turn increases the ionicity of chemical bonds of glass. Hence it increases the intermolecular free space which leads to increase the glass molar volume [21].
. As shown in Fig. 4, increasing the mole fraction contents of SeO2 and Cr2O3 together up to 0.006 (sample 10) increases the free space per mole from 21.14 (sample 1) to 21.75 cm3/mole, respectively. So the conjunct introduction of SeO2 and Cr2O3 enhances the 2p electron density of the unshared oxygen ion which in turn increases the ionicity of chemical bonds of glass. Hence it increases the intermolecular free space which leads to increase the glass molar volume [21].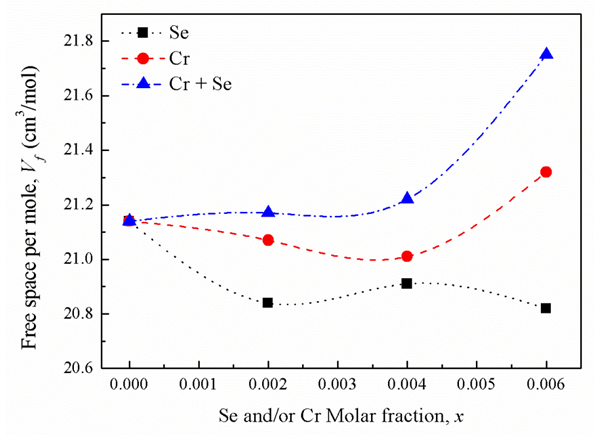 | Figure 4. Free space per mole versus oxide molar fractions |
3.5. Molar Volumeand the Bond Ionicity
- Boron has ionic cation radius 0.20 Å while Se and Cr have relative large ionic cation radii 1.84 and 0.65 Å, respectively. Therefore, it is expected that, with the insertion of Se and Cra transformation of a part of BO3 groups into BO4 groups is taking place. It produces an assured increase in the number of nonbridging oxygen bonds (NBO) relative to the bridging bonds (BO), which manifests itself by the increase in molar volume. Furthermore, the ionicity of oxides decreases with increasing polarization power of cations [22]. Accordingly, ionic character or bond ionicity (Ib) could describe the bonding nature of the investigated glass [23]. Therefore, the change in the molar volume of the present glass system could be discussed on the basis of a number of parameters such as electronegativity difference and Pauling’s ionicity. The glass electronegativity difference, Δχi, is calculated using the formula:
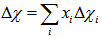 | (10) |
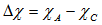 | (11) |
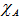 and
and 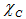 are the Pauling electronegativity of the anion (oxygen) and that of the cation, respectively. Applying Pauling’s empirical definition of bond ionicity, Ib, where
are the Pauling electronegativity of the anion (oxygen) and that of the cation, respectively. Applying Pauling’s empirical definition of bond ionicity, Ib, where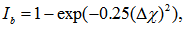 | (12) |
3.6. Nearest-neighbor Coordination
- Because of its large glass forming region, nearest-neighbor coordination in a quaternary glass system is mostly appropriate to explain the obtained glass molar volume values. The average coordination number, m, in the present glass system is defined by
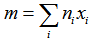 | (13) |
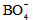 units where the negative charge is distributed around the boron site. The second is due to the conversion from bridging to nonbridging oxygen bonds which increases the negative charge localized on the B-O bond with a deeper potential well. The third is due to conversion of chromium ions from Cr6+ state to Cr3+ state. Thus through the mixed valence effect, with 3d-4p orbital replacement of B2O3 by a combination of small conjoint molar fractions of Cr2O3 and SeO2, the molar volume of a heavy metal glass could be tuned. Thus it is possible to make comparatively cheap junctions with important implications for the economics of solar cells. No doubt, this anionic conduction would motivate the research on chalcogen-oxide glasses for Li-ion batteries, fluorescent lights, cellular phones, and fuel cells. So, increasing the glass molar volume might contribute directly in the production of promising glasses for ionic conduction domain [18, 27-29].
units where the negative charge is distributed around the boron site. The second is due to the conversion from bridging to nonbridging oxygen bonds which increases the negative charge localized on the B-O bond with a deeper potential well. The third is due to conversion of chromium ions from Cr6+ state to Cr3+ state. Thus through the mixed valence effect, with 3d-4p orbital replacement of B2O3 by a combination of small conjoint molar fractions of Cr2O3 and SeO2, the molar volume of a heavy metal glass could be tuned. Thus it is possible to make comparatively cheap junctions with important implications for the economics of solar cells. No doubt, this anionic conduction would motivate the research on chalcogen-oxide glasses for Li-ion batteries, fluorescent lights, cellular phones, and fuel cells. So, increasing the glass molar volume might contribute directly in the production of promising glasses for ionic conduction domain [18, 27-29]. 4. Conclusions
- Conduction band mainly consists of atomic orbitals of cationic atoms, since anionic atoms are more electronegative than cationic atoms. Upon the structure of investigated glass, the p- and d- orbitals strongly interact so that the d-states of the transition metal atoms Cr hybridize with the chalcogen Se p-bands. Such orbital hybridizationis basically corresponding to nonbonding states with more optical polarization. Depending on the nature of valence orbitals, the substitution of p- and d-block elements results as increase in the glass molar volume through creation of nonbridging oxygen bonds. The looked-for mixed valence effect (through a very small replacement of boron oxide content by an equal share of molar fractions of SeO2 and Cr2O3) is to replace strong oxygen linkages of triagonal
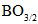 units by weaker anionic tetrahedral
units by weaker anionic tetrahedral 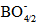 unit linkages and the more polarizable Cr3+ state. The projected result would be glass network weakening with a decrease in both the melting temperature and the viscosity of the melt. Due to the weaker bonding, the molar volume increases and the glass network becomes more open. It provides wide pathways get ways for ions such as Li+ to be more easily mobile in the glass matrix. The orbital hybridization is proven itself as an active technique to control the glass molar volume and thus to regulate effectively the glass ionic conduction. The gained results would encourage and may drive to further prospective researches on the fields of interest related to the present study.
unit linkages and the more polarizable Cr3+ state. The projected result would be glass network weakening with a decrease in both the melting temperature and the viscosity of the melt. Due to the weaker bonding, the molar volume increases and the glass network becomes more open. It provides wide pathways get ways for ions such as Li+ to be more easily mobile in the glass matrix. The orbital hybridization is proven itself as an active technique to control the glass molar volume and thus to regulate effectively the glass ionic conduction. The gained results would encourage and may drive to further prospective researches on the fields of interest related to the present study. ACKNOWLEDGEMENTS
- This work is supported by the National Research Centre, Egypt project no. 10070402.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML