-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2014; 4(2): 111-117
doi:10.5923/j.materials.20140402.08
Inhibitive Behaviour of Methyl Red (2, 4-Dimethylamino-2'-carboxylazobenzene) on Corrosion of Mild Steel in Alkaline Medium
S. I. Durowaye1, O. I. Sekunowo1, V. O. Durowaye2
1Department of Metallurgical and Materials Engineering, University of Lagos, Akoka, Lagos, Nigeria
2Department of Chemistry, International School, University of Lagos, Akoka, Lagos, Nigeria
Correspondence to: S. I. Durowaye, Department of Metallurgical and Materials Engineering, University of Lagos, Akoka, Lagos, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
The corrosive behaviour of mild steel in 1M NaOH solutions contaminated with methyl red (2, 4-Dimethylamino-2'-carboxylazobenzene), an organic compound at room temperature was studied using the weight loss technique. The concentration of the methyl red was varied as 1.0%, 2.0%, 3.0%, 4.0%, 5.0% and 6% weight of each alkaline medium and their effects were carefully studied on the corrosion rate of mild steel. The results obtained showed methyl red to be an efficient inhibitor in the alkaline environment with general decrease in corrosion rate as the concentration of the organic compound increases. The Langmuir adsorption isotherm was found to be the best description of the adsorption behaviour of Methyl red on the mild steel surface.
Keywords: Corrosion, Mild Steel, Methyl Red, NaOH, Optical Emission Spectrometer
Cite this paper: S. I. Durowaye, O. I. Sekunowo, V. O. Durowaye, Inhibitive Behaviour of Methyl Red (2, 4-Dimethylamino-2'-carboxylazobenzene) on Corrosion of Mild Steel in Alkaline Medium, American Journal of Materials Science, Vol. 4 No. 2, 2014, pp. 111-117. doi: 10.5923/j.materials.20140402.08.
Article Outline
1. Introduction
- Metallic material constitutes a great part of construction material elements in industries, agricultural equipment, oil and gas and petrochemical, medical services, process and allied industries. In these industries, the metallic material as a result of interaction with its environment loses its integrity over a period of time [1]. As such, the material cannot perform the intended function effectively and reliably. Some of these environments are atmosphere, aqueous solution, solids, acids and bases, inorganic solvents, molten salts, liquid metals, human body etc. At times, the effect of the loss in integrity may be very severe as to result in loss of valuable production time, accident and in the extreme death. The cost associated with the problem is enormous and its influence in the economy life of a nation is significant. It has been estimated that approximately 5% of industrialised nations’ income is spent on corrosion incidental problems. For instance, the cost of the problem to the U.S. economy is put at $297 billion [2]. The cost to the Nigeria economy is not available. However, a guess could be haphazard; and this is in the corridor of $3.2 billion [3].In frequent instances the addition of small quantities of special chemicals to large volumes of corrosive solutions can be quite effective in reducing corrosion. Such chemicals are called corrosion inhibitors. At the simplest level a corrosion inhibitor is a substance which when added in small concentration to an environment effectively reduces the corrosion rate of a metal exposed to that environment. Because of the toxic nature and high cost of some chemicals used like chromate and nitrate, it is necessary to develop environmentally acceptable and less expensive inhibitors [4]. Organic inhibitors in particular are of great use in the control of corrosion in sea water. Mild steel corrosion in acidic solution has also been effectively controlled by the use of organic substances containing nitrogen, oxygen or sulphur in the conjugated system as inhibitors [5]. Successful use of naturally occurring inhibitors in both acidic and alkaline environments has been extensively reported [6]. This method of corrosion control is fraught with the challenges of high cost and toxicity. The efficiency of several synthetic organic compounds such as methylsulfiny, carbanion-dimethyl sulfoxide, hydroxyl radicals and alkyl nitrates has been investigated [7]. Corrosion mitigation of structures by these synthetic compounds is significant. However, the known hazardous effects of most synthetic organic inhibitors still pose a great challenge. Most of the efficient inhibitors used in industry are organic compounds having multiple bonds in their molecules which mainly contain nitrogen and sulphur atoms through which they are adsorbed on the metal surface. Compounds with functional groups containing oxygen, nitrogen and sulphur have ability to form complexes with iron. They have been reported to act as effective inhibitors to the surface of steel by means of their competitive adsorption through the surface complex formation [8].Organic compounds used as inhibitors act through a process of surface adsorption, so the efficiency of an inhibitor depends on (i) the chemical structure of the organic compound, (ii) the surface charge of the metal, and (iii) the type of interactions between the organic molecule and metal surface. Existing data reveal most inhibitors to act by adsorption on the metal surface through heteroatoms such as nitrogen, oxygen and sulphur, double bonds, triple bonds or aromatic rings which tend to form stronger coordination bonds. Compounds with π-bonds generally exhibit good inhibitive properties, the electrons for the surface interaction being provided by the π-orbital [9]. The corrosion inhibition of steels has received a considerable amount of attention as a result of its industrial concern [10, 11]. Steel passivation in alkaline environments is due to the formation of a very thin, but highly protective oxide/hydroxide layer [12]. It has been proved that, in alkaline media, the film corresponds basically to a double-layer model consisting of an inner magnetite and an outer ferric oxide according to a Fe3O4/ Fe3+ structure [13 – 19]. The most internal layer is composed of Fe2+ oxides in contact with the substrate. The thermodynamic instability of both Fe2+ oxides and magnetite in the presence of oxygen leads to the formation of an outer layer of Fe3+ oxides and continuous exposure to oxygen and humidity promotes film growth and a progressive enrichment of the passive film in Fe3+, either in oxide form or oxo-hydroxide form, depending on the potential. The passivity breakdown occurs by addition of Cl- to the alkaline solution [20]. In practice, corrosion can never be stopped but can be hindered to a reasonable level. Among many methods of corrosion control and prevention, the use of organic inhibitors is the most frequently used. Corrosion control is an important activity of technical, economical and environmental importance. Thus, the search for efficient corrosion inhibitors has become a necessity to secure metallic materials against unmitigated degradation. Corrosion of mild steel is of great practical interest because mild steel is widely used in the oil, gas and offshore environments for pipelines, flow-lines, platforms, down-hole tubular equipments, well heads, industrial vessels etc. Corrosion inhibition is being extensively employed in minimising metallic wastage of engineering materials in service [21]. In this research, the effect of the inhibition efficiency of methyl red on the corrosion of mild steel in alkaline medium using 1M sodium hydroxide solution (NaOH) is experimentally investigated and presented.
2. Experimental Details
2.1. Work Materials and Specimen Preparation
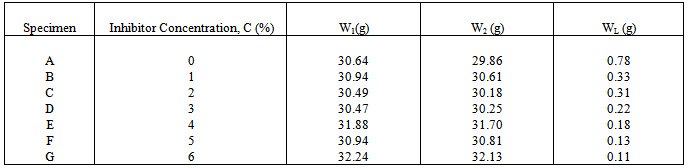
- The cylindrical mild steel rod used for this study was obtained in the open market and analysed at the Research Laboratory, Department of Metallurgical & Materials Engineering, University of Lagos, Akoka, Nigeria. It was cut to a length of 30cm and properly machined to diameter 15mm. It was manually polished using silicon carbide grinding papers of grit 60 and 120 to ensure a smooth surface and reduce corrosion attack on the specimen. After polishing, the rod was cut into lengths of 5.25cm and the edges of these cut pieces were also polished. The rods were kept in a desiccator to prevent corrosion prior to experimentation. Seven specimens of length 5.25cm and diameter 15mm were used for the experiment and were denoted as A, B, C, D, E, F and G.
2.2. Inhibitor and Alkaline
- Methyl Red (2, 4-Dimethylamino-2'-carboxylazobenzene), a reddish powder is the inhibitor used. Its structural formula is shown in figure 1 and its molecular formula is C15H15N3O2. It was prepared by dissolving 0.1g of Methyl red in 75ml of 95% ethanol and distilled water was added to make 100 ml. Various concentrations of 1%, 2%, 3%, 4%, 5% and 6% weight of each alkaline medium were used as the inhibiting compound. 40g of NaOH was dissolved in 1000cm3 of distilled water to get 1M NaOH. The NaOH used as corrosive medium was from Analar grade and bi-distilled water was used for its preparation.
 | Figure 1. Chemical structure of Methyl Red (2, 4-Dimethylamino-2'-carboxylazobenzene) |
2.3. Test Procedures
|
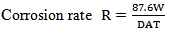 | (1) |
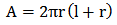 | (2) |
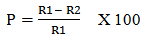 | (3) |
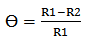 | (4) |
3. Results and Discussion
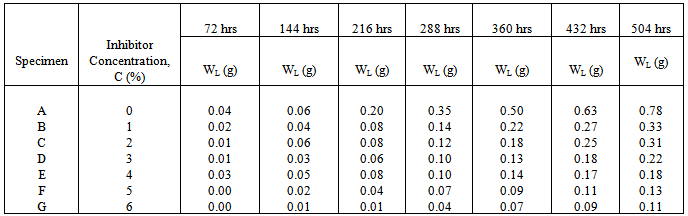
- The spectrochemical analysis shown in table 1 reveals that the mild steel specimens used in this study have an iron content of approximately 99% with other trace elements residued from metal extraction process. The levels of these trace elements are insignificant to influence the chemistry of the behaviour of the steel specimens in the corrosive medium.
|
|
|
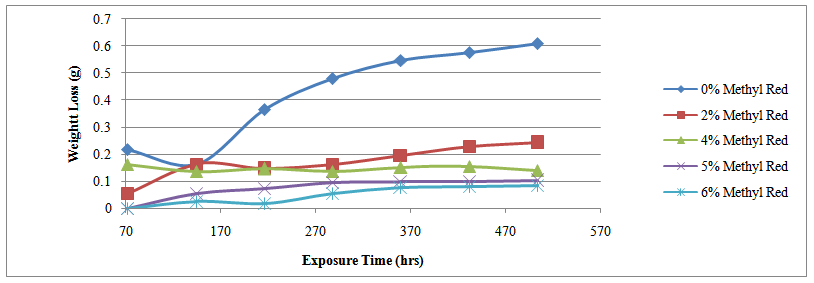 | Figure 2. Variation of weight-loss with exposure time for specimens A, C, E, F & G in 0%, 2%, 4%, 5% & 6% Methyl red concentrations |
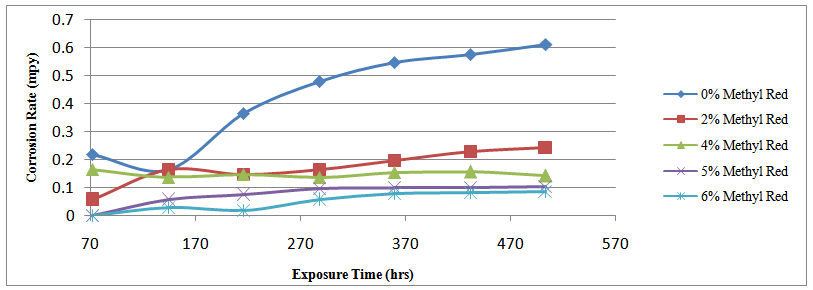 | Figure 3. Effect of percentage concentration of Methyl red on the corrosion rate of mild steel specimen A, C, E, F & G |
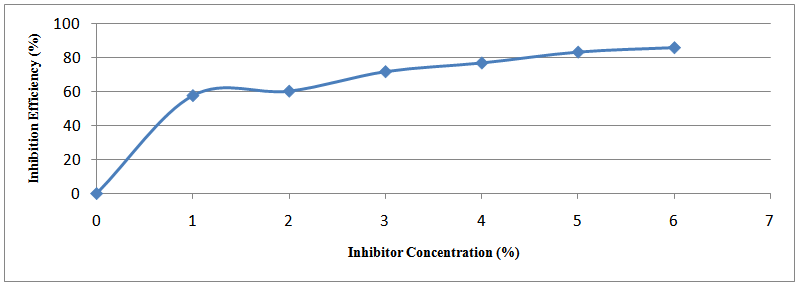 | Figure 4. Variation of percentage inhibition efficiency with Methyl red at concentrations at 504 hrs |
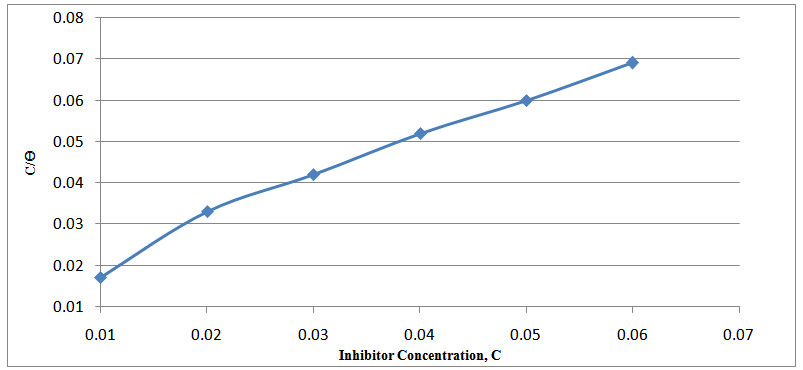 | Figure 5. Langmuir isotherm adsorption model on the Mild steel surface in 1M NaOH solution containing different concentrations of inhibitor at 504 hrs |
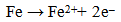 | (5) |
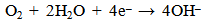 | (6) |
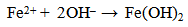 | (7) |
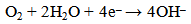 | (8) |
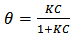 | (9) |
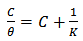 | (10) |
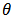 the surface coverage of the inhibitor. The linear relationship of
the surface coverage of the inhibitor. The linear relationship of 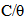 versus C in different concentration of Methyl red solutions is displayed in Figure 5. However, the slope of the relation shows a little deviation from unity, this might be the result from the interactions between the adsorbed species on the metal surface [33, 34]. The K value may be taken as a measure of the strength of the adsorption forces between the inhibitor molecules and the metal surface [35].
versus C in different concentration of Methyl red solutions is displayed in Figure 5. However, the slope of the relation shows a little deviation from unity, this might be the result from the interactions between the adsorbed species on the metal surface [33, 34]. The K value may be taken as a measure of the strength of the adsorption forces between the inhibitor molecules and the metal surface [35]. 4. Conclusions
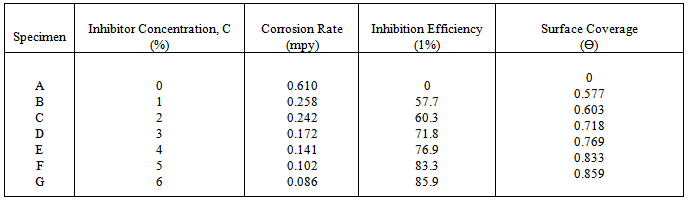
- In this study, the weight loss technique reveals Methyl red to be an efficient inhibitor for mild steel in the sodium hydroxide corrosive medium. The inhibition efficiency increases with the increase in concentration of Methyl red with maximum inhibition efficiency value of 85.9% at 6% inhibitor concentration. At this value, the lowest corrosion rate value of 0.086 mpy was obtained. This is a mixed type inhibitor whose adsorption on mild steel is physiochemical. It mainly acts by film formation, thus blocking the active sites on the cathodic and anodic regions. The adsorption of this inhibitor obeys Langmuir’s adsorption isotherm.
ACKNOWLEDGEMENTS
- The authors would like to thank Professor G.I. Lawal, Head of Department, Metallurgical and Materials Engineering Dept, University of Lagos for his tremendous support and Mr. B.M. Begusa, Former Chief Technologist, Chemistry Department, University of Lagos, for his valuable suggestions.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML