-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2014; 4(2): 97-102
doi:10.5923/j.materials.20140402.06
Isolation and Characterization Studies of Ginger (Zingiber officinale) Root Starch as a Potential Industrial Biomaterial
Michael O. Afolayan1, Kenneth K. Adama2, Anthony Oberafo2, Moses Omojola3, Sunday Thomas1
1Chemistry Advanced Laboratory, Sheda Science & Technology Complex, P. M. B. 186, Garki – Abuja, Nigeria
2Physics Advanced Laboratory, Sheda Science & Technology Complex, P. M. B. 186, Garki – Abuja, Nigeria
3Raw Materials Research and Development Council, PMB 232, Garki – Abuja, Nigeria
Correspondence to: Michael O. Afolayan, Chemistry Advanced Laboratory, Sheda Science & Technology Complex, P. M. B. 186, Garki – Abuja, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Ginger (Zingiber officinale) roots were crushed and the starch extracted in order to determine its starch composition and use in biomaterials development as hybrid composites. The physicochemical properties of the isolated starch were then determined and compared with standard industrial maize starch. The starch was isolated using 1% w/v sodium metabisulphite solution and the obtained starch was found to be a white, crystalline, non- hygroscopic powder with yield of about 18%. The starch percentage solubility at 90℃ was 1.87 with a swelling power of 11.07 and gelatinization temperature of 78℃. It had a browning temperature of 243.5 – 248.2℃, charring temperature of 278.6 – 285.2℃, water absorption capacity of 90%, pH of 6.54, foam and emulsion capacities of 2.5% and 5.7% respectively. XRD analysis of the starch sample at 10℃ – 100℃ 2Θ angle showed the starch to be a polycrystalline organic polymer of pharmaceutical/medicinal grade. The percentage composition of amylose to amylopectin was found to be in the ratio of 32% to 68% with an orthorhombic crystal structure. The profile revealed peak positions at 2Θ positions of 11.2282°, 15.0054°, 17.0346°, 18.0121° and 22.8782° corresponding to a Full Width at Half Maximum (FWHM) (2Θ) of 0.7911°, 0.5274°, 0.4615°, 0.6593° and 0.5934° respectively. The spectral of the ginger starch sample corroborate with standard corn starch which served as the reference with a quality score of 82%. Applicability in composite materials studies showed a high level of compatibility as binder/filler materials within the matrix and fibre materials employed. Generally, the values obtained from the characterization of ginger starch compared favourably with corn starch and showed that it has high potential for industrial applications as biomaterials in composites, food, textile and pharmaceutical industries.
Keywords: Ginger, Starch, Physicochemical, XRD, Composites, Biomaterial
Cite this paper: Michael O. Afolayan, Kenneth K. Adama, Anthony Oberafo, Moses Omojola, Sunday Thomas, Isolation and Characterization Studies of Ginger (Zingiber officinale) Root Starch as a Potential Industrial Biomaterial, American Journal of Materials Science, Vol. 4 No. 2, 2014, pp. 97-102. doi: 10.5923/j.materials.20140402.06.
Article Outline
1. Introduction
- Starch is one of the most abundant organic chemicals on earth. It is found in the leaves of green plants in the plastids where it is synthesized. It is also synthesized in the amyloplasts of seeds, grains, roots and tubers of most plants where it serves as the chemical storage form of energy [1]. Starch is a natural biodegradable biopolymer which has wide industrial applications. Starch is the only qualitatively important digestible polysaccharide and has been regarded as nutritionally superior to low molecular weight carbohydrate or sugars [2]. Though starch is mainly used as food, it can also be readily converted chemically and biologically into many useful and diverse products such as paper, textiles, adhesive beverages, confectionaries, pharmaceuticals and plastics [3]. Starches from different sources have been evaluated and use as an excellent binder and pharmaceutical excipients in either mucilage or dry powder form. There are many potential uses of starch industrially: unmodified starch can be used in the pharmaceutical, paper, mining and building industries. It can be modified and converted to starch derivatives [4]. Starch is also one of the most widely used biomaterial in the food, textile, cosmetics, plastics, adhesives, paper and pharmaceutical industries. The diverse industrial usage of starch is based on its availability at low cost, high calorific value and inherent excellent physicochemical properties. The versatility of starch in industrial applications is clearly defined by its physicochemical properties; therefore, a thorough evaluation of the necessary parameters is important in elucidating its industrial uses. As a result of the competing demands for starch as food, pharmaceutical and industrial uses coupled with the need to attain self sufficiency in starch production, there is a need to find other high yield sources different from cassava, maize and potato [5]. Ginger (Zingiber officinale) is an herbaceous perennial plant belonging to the order Scitamineae and the family Zingiberaceae. It is a perennial reed – like plant with annual leafy stems about a meter (3 – 4 feet) tall. It is a root crop and a typical herb extensively grown across the world for its pungent aromatic under-ground stem or rhizome which makes it an important export commodity in world trade [12]. Ginger root is the rhizome of the plant Zingiber officinale, consumed as a delicacy, medicine or spice. It is from the family Zingiberaceae. Ginger cultivation began in South Asia and has since spread to East Africa and the Carribean [6]. Ginger was introduced to Europe by Arab traders from Indian the first century AD. The Arabs also took the plant from India to East Africa in the thirteenth century while the Portuguese took it to West Africa and other part of the tropics in the sixteen century. Ginger was introduced to Nigeria in 1927 [13]. The plant is now cultivated in different parts of Nigeria, though the major producing areas include Kaduna, Nasarawa, Sokoto, Zamfara, Akwa Ibom, Oyo, Abia and Lagos states although southern Kaduna still remain the largest producers of fresh ginger in Nigeria in Kachia, Jabba, Jama’a and Kagarko Local Government Areas [14]. The quality of Nigeria Ginger being the best in the world has contributed to and is an indicator of the increased demand for Ginger.During the present study, starch was extracted from the roots of ginger and its physicochemical and other functional properties evaluated and compared with that of standard industrial maize starch.
2. Experimental
2.1. Materials
- Ginger roots were bought from Gwagwalada market, F.C.T., Nigeria. Corn starch (BP) and other analytical grade reagents were obtained from Chemistry Advanced Laboratory, Sheda Science and Technology Complex, Abuja Nigeria.
2.2. Starch Isolation
- The fresh ginger roots were peeled and washed. Peeled roots (2.106 kg) were chopped into small pieces and soaked in sodium metabisulphite solution (2 L 1% w/v) at room temperature (27℃). Thereafter, the chopped roots were removed and wet milled into a slurry using a grater. The paste was dispersed in a large volume of 1% sodium metabisulphite solution and filtered through muslin cloth. The suspension was centrifuged at 3500 rpm for 10 mins to facilitate the removal of dirts. The supernatant was carefully decanted and the mucilage scraped off. The process was repeated for three times with the mucilage on the starch scraped continuously until a pure starch was obtained. The resulting starch was dried in the sun and further dried at 60℃ in a hot air oven, pulverized, weighed and stored in sample bottles for analysis.
2.3. Determination of Certain Physicochemical Properties
2.3.1. Swelling Power
- The method described by Afolayan et al (2012) was used to determine the solubility index. The starch sample (0.1 g) was weighed into a test tube and 10 ml of distilled water was added. The mixture was heated in a water bath at a temperature of 50℃ for 30 min with continuous shaking. In the end, the test tube was centrifuged at 1500 rpm for 20 min in order to facilitate the removal of the supernatant which was carefully decanted and weight of the starch paste taken. The swelling power was calculated as follows:
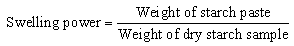 This was carried out over a temperature range of 50℃ – 100℃.
This was carried out over a temperature range of 50℃ – 100℃.2.3.2. Solubility Index
- The method described by Afolayan et al (2012) was also used to determine the solubility index. Starch sample (0.5 g) was added to 10 ml distilled water in a test tube. This was subjected to heating in a water bath with a starting temperature of 50℃ for 30 min. It was then centrifuged at 1500 rpm for 30 min. 5 ml of the supernatant was decanted and dried to constant weight. The solubility was expressed as the percentage (%) by weight of dissolved starch from heated solution. This was carried out over a temperature range of 50℃ – 100℃.
2.3.3. pH
- A 20% w/v dispersion of the sample was shaken in water for 5 minutes and the pH was determined using a pH meter.
2.3.4. Browning and Charring Temperature
- The method of Builders et al (2001) was used. Some of the starch sample was put into a capillary tube, the browning and charring temperatures were determined using a melting point apparatus with model Electrothermal 9100.
2.3.5. Foam Capacity
- The method of Omojola et al (2010) was used with slight modifications. Starch sample (1 g) was homogenized in 50 ml distilled water using a vortex mixer (vortex 2 Genie set at shake 8) for 5 minutes. The homogenate was poured into a 100 ml measuring cylinder and the volume recorded after 30 s. The foam capacity was expressed as the percent increase in volume.
2.3.6. Emulsion Capacity
- Sample (1 g) was dispersed in 5 ml distilled water using a vortex mixer for 30 seconds. After complete dispersion, 5 ml vegetable oil (groundnut oil) was added gradually and the mixing continued for another 30s. The suspension was centrifuged at 1600 rpm for 5min. The volume of oil separated from the sample was read directly from the tube. Emulsion capacity is the amount of oil emulsified and held per gram of sample.
2.3.7. Gelatinization Temperature
- This was evaluated using the method of Attama et al (2003). The starch sample (1g) was put in a 20 ml beaker and 10 ml of distilled water was added. The dispersion was heated on a hot plate. The gelatinization temperature was then read with a thermometer suspended in the starch slurry.
2.3.8. Water Absorption Capacity
- The method described by Omojola et al (2010) was used to determine the water absorption capacity. The starch sample (5% w/v) was dispersed in a pre-weighed centrifuge tube. The tube was agitated in a vortex mixer for 2 min. The supernatant was then discarded and the weight of the tube and hydrated sample taken. The weight was calculated and expressed as the weight of water bound by 100 g dry starch.
2.3.9. Structural Analysis of the Ginger Root Starch Samples
- The XRD spectra were obtained by means of PANalytical XPERT PRO MPD x-ray diffraction PW 3040/60 system using CuKα monochromatic radiation. The wavelength, accelerating voltage and current were 1.5418A, 40KV and 30mA respectively. The prepared powdered samples were loaded onto a cylindrical sample holder which was then mounted onto the XRD machine sample stage. A monochromatic beam of light was allowed to strike the mounted sample after spinning was enabled on the sample stage for the required scanning time and 2Θ angle. The intensity of the rays was measured at Bragg’s 2Θ angle from start angle of 10° to end angle of 100° and this was displayed as the scan spectral on a real time computer connected to the XRD system. The crystallinity and other important parameters were determined from the x-ray diffraction analysis using the system’s softwares.
3. Results and Discussions
3.1. Chemical Composition
- Table 1 shows some physicochemical properties of the starch. The starch obtained was a white, crystalline, non- hygroscopic powder with no smell and a yield of about 18%. The yield is considered to be appreciable especially when compared with starches from other sources such as cassava and corn.
3.2. Swelling and Solubility
- The swelling and solubility profiles of ginger and maize starches over a temperature range of 50 – 100℃ are shown in Figures 1 and 2.
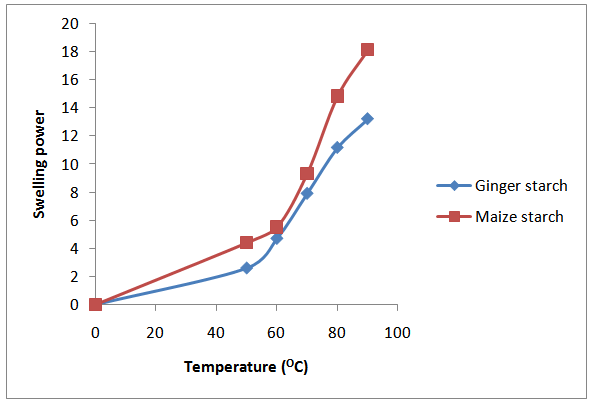 | Figure 1. Swelling Profile for Maize and Ginger Starch |
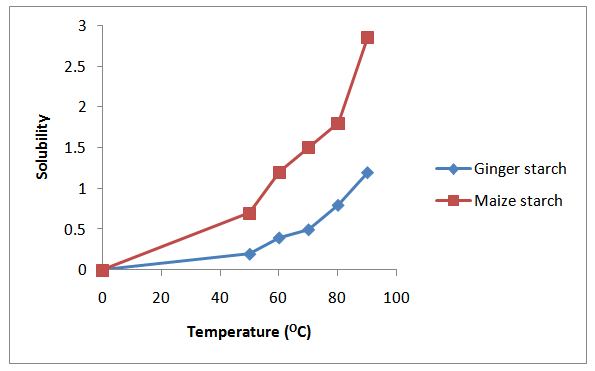 | Figure 2. Solubility Profile For Maize And Ginger Starch |
3.3. Structural Analysis by XRD
- XRD analysis of the ginger root starch samples indicated prominent peaks at 2θ positions of 11.2282°, 15.0054°, 17.0346°, 18.0121° and 22.8782°. The most intense peak was at 2θ position of 17.0346° corresponding to a FWHM of 0.4615°, peak height of 2646.44cts, d-spacing of 5.20524A and relative intensity of 100%. The XRD spectrum of the ginger root starch sample is shown in figure 3.
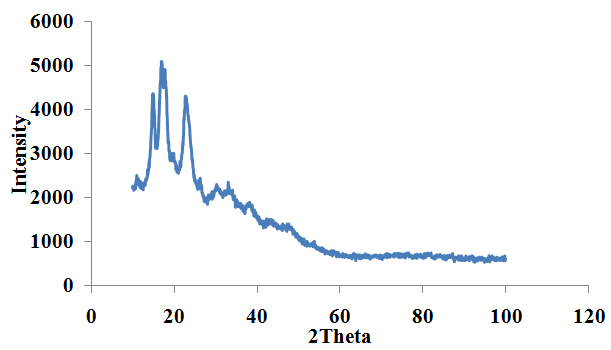 | Figure 3. Ginger starch |
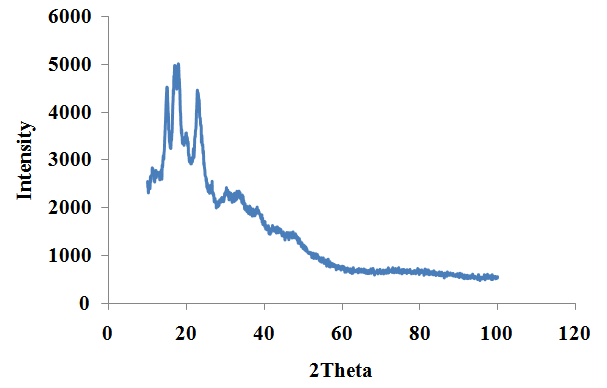 | Figure 4. Standard Maize Starch |
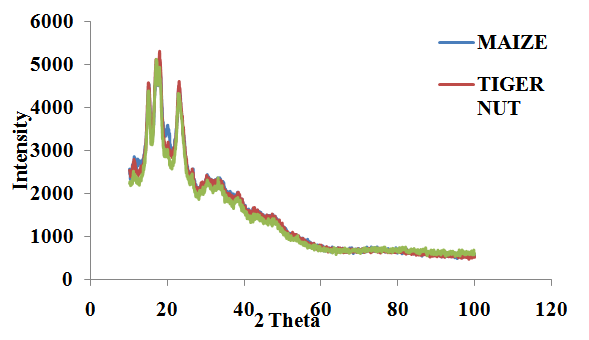 | Figure 5. Tigernut, Maize and Ginger Starch |
4. Conclusions
- Some physicochemical properties of ginger starch have been examined and these properties compare favourably with other starches especially maize starch. The study has therefore shown that ginger starch may be a good source of starch and a biomaterial for industrial uses. Therefore it is a potential source of industrial starch. However, further characterization and starch conversion studies need to be carried out to fully predict and maximize the industrial potentials of ginger starch.
References
| [1] | Afolayan, Michael O., Omojola, Moses O., Orijajogun, Joyce O. , Thomas, Sunday A. (2012): Further physicochemical characterization of Anchomanes difformis starch. Agriculture and Biology Journal of North America 3(1): 31 –38. |
| [2] | Ogungbenle H. N. (2007): Effect of chemical modification on starch of some legume flours; Pakistan Journal of Nutrition 6 (2): 167-171. |
| [3] | Agbo I. U., and Odo G. E. (2010): The effect of hypochlorite oxidation and acetylation on some of the physicochemical properties of icacina trichantha starch; Bio – Research 8 (1): 593 – 597. |
| [4] | Omojola M. O., Akinkunmi Y. O., Olufunsho K. O., Egharevba H. O. And Martins E. O. (2010): Isolation and physico-chemical characterization of cola starch; African Journal of food, agriculture, nutrition and development. 10(7):2884 – 2900. |
| [5] | Gebre – Mariam T., Schmidt P. C. (1996): Isolation and physicochemical properties of Endset starch; Starch / Starke 48 (6), 208 – 214. |
| [6] | “Spices: Exotic flavours and medicines – Ginger” http://unitproj.library.ucla.edu/biomed/spice/index.cfm?displayID=15 Retrieved 8 August 2007. |
| [7] | Coursey D. G. And Rasper V. (1967): Properties of starches of some west African yams; J. Sci. Food Agric., 18: 240 – 248. |
| [8] | Builders P. E., Emeje M. And Kunle O.O (2001): Some physico – chemical properties of cyperus starch – A potential pharmaceutical excipient; Journal of Pharmaceutical and Allied Sciences 2. |
| [9] | Chowdary K. P. R. & Enturi V. (2011): Preparation, characterization and evaluation of starch citrate- a new modified starch as a disintegrant in tablet formulations; International Journal of Pharm. Research and Development 12 (2) 9 – 17. |
| [10] | Nuwamanya E., Baguma Y., Emmambux N., Taylor J. and Rubaihayo P. (2010): Physicochemical and functional characteristics of cassava starch in Ugandan varieties and their progenies; Journal of Plant Breeding and Crop Science 2 (1) 001 – 011. |
| [11] | Attama A. A., Nnamani P. O., Mbonu I. K. and Adiku M. U. (2003): Effect of hypochlorite oxidation on the physicochemical properties of gladiolus starch; Journal of Pharm and allied Science 1 (1) 28-35. |
| [12] | Erinle, I.D. (1988). An Overview of Research on Ginger Productions in Northern State of Nigeria. Proceedings of the First National Ginger Workshop, Umudike, Nigeria, 89-93. |
| [13] | Bernard,A.(2008). Diseases, pest and other factors limiting ginger (Zingiberofficinale Rose) production in River State. Being the text of a paper delivered during the Agricultural Product Development Strategy Workshop organized by Uptonville Foundation under the aegis of Rivers State Sustainable Development Agency(RSSDA). Retrieved from http://uptonvilleoginstu.org/ginger.litm. |
| [14] | KADP (Kaduna State Agricultural Development Project) (2000). Production of ginger: an extension guide. Kaduna State Agriculture Development Project, Kaduna. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML