-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2013; 3(5): 110-119
doi:10.5923/j.materials.20130305.02
Fabrication and Characterisation of Ghanaian Bauxite Red Mud-Clay Composite Bricks for Construction Applications
D. Dodoo-Arhin, D. S Konadu, E. Annan, F. P Buabeng, A. Yaya, B. Agyei-Tuffour
Department of Materials Science and Engineering, University of Ghana, P. O. Box Lg 77, Legon-Accra, Ghana
Correspondence to: D. Dodoo-Arhin, Department of Materials Science and Engineering, University of Ghana, P. O. Box Lg 77, Legon-Accra, Ghana.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The behaviour of Ghanaian based bauxite red mud-Tetegbu clay composites have been investigated for their applicability in the ceramic brick construction industry as a means of recycling the bauxite waste. The initial raw samples were characterized by X-ray diffraction (XRD), X-ray Fluorescence spectroscopy (XRF), Fourier transform infrared spectroscopy (FTIR), and thermogravimetric analysis (Tg-DTA). The red mud-clay composites have been formulated as 80%-20%, 70%-30%, 60%-40%, 50%-50% and fired at sintering temperatures of 800ºC, 900ºC and 1100ºC. Generally, mechanical strengths (modulus of rupture) increased with higher sintering temperature. The results obtained for various characterization analyses such as bulk densities of 1.59 g/cm3 and 1.51 g/cm3 compare very well with literature and hold potential in bauxite residue eco-friendly application for low-cost recyclable constructional materials.
Keywords: Red Mud-Clay Bricks, Bauxite Residue, Modulus of Rapture, XRD, Thermal Analysis
Cite this paper: D. Dodoo-Arhin, D. S Konadu, E. Annan, F. P Buabeng, A. Yaya, B. Agyei-Tuffour, Fabrication and Characterisation of Ghanaian Bauxite Red Mud-Clay Composite Bricks for Construction Applications, American Journal of Materials Science, Vol. 3 No. 5, 2013, pp. 110-119. doi: 10.5923/j.materials.20130305.02.
Article Outline
1. Introduction
- In recent times, the exploration of recycling alternatives for several industrial wastes or by-products has become a common practice. Some few industries however do not adhere to proper environmental regulations well leading to environment pollution. Recycling procedures are usually conducted under the influence of legislation or as a means of eliminating disposal cost and avoiding soil and water pollution. In the building industry, recycling of waste materials is environmentally friendly as these materials can be re-use as starting materials for engineering applications. Many of the industrial waste products however, usually contain substantial amounts of inorganic ingredients, such as silicon, aluminium, calcium and iron oxides[1] One such industrial waste product is the red mud; the main solid by-product in the production of alumina by alkaline extraction routine from bauxite ore via the Bayer process. Bauxite consists of about 75 % of hydrated alumina (Al2O3·3H2O and Al2O3·H2O). During the treatment of the bauxite ore by the Bayer process (figure 1), it is initially crushed and then mixed with a hot solution of sodium hydroxide, NaOH, at ≈175 °C and lime liquor and subjected to attack at high pressure and temperature. This condition makes it possible to convert the hydrated alumina and to obtain sodium aluminate solution (Eq. (1)), while the impurities remain in a solid state.
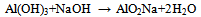 | (1) |
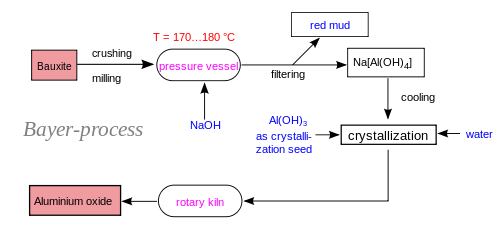 | Figure 1. The Bayer process for red mud and alumina production |
2. Experimental
2.1. Materials and Methods
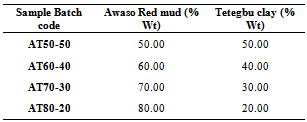
- Sample PreparationThe Awaso bauxite sample for the red mud production and Tetegbu clay samples were obtained from the Western and Greater Accra regions of Ghana respectively as shown in figure 2.In a typical red mud production via the Bayer process, bauxite samples were reduced to fine particle size of ≈100µm by milling using a Thomas ball-mill machine and granulometer. The milled bauxite was digested in a 1000ml Pyrex volumetric flask containing a hot (135°C - 140 °C) 2M NaOH solution for about 30minutes under constant stirring to allow even dispersion. The homogenous mixture was then allowed to cool to ambient temperature and the particles allowed to sediment. The liquid phase was then decanted and the residue (red mud) dried in an autoclave at 110ºC for ≈ 48 hours. The samples were then allowed to cool overnight to room temperature.Characterization of the Bauxite, Clays and Red mudX-Ray Fluorescence mineralogical composition of the samples was determined using a Thermo Fisher ARL9400 XP+ Sequential XRF equipped with a WinXRF software was for analyses. The samples were milled in a tungsten-carbide milling pot to achieve particle sizes <75micron. The samples were dried at 100°C and roasted at 1000°C to determine Loss on Ignition (LOI) values. 1g Sample was mixed with 6g lithium tetraborate flux (Li2B4O7) and fused at 1050°C to make a stable fused glass bead. For trace element analyses the sample was mixed with a PVA binder and pressed into a pellet using a 10 ton press.X-ray powder diffraction (XRD) patterns were collected on an XPERT-PRO diffractometer (PANalytical BV, Netherlands) with theta/theta geometry, operating a cobalt tube at 35 kV and 50 mA. The goniometer is equipped with automatic divergence Slit and a PW3064 spinner stage. The XRD patterns of all specimens were recorded in the 5.0°- 90° 2θ range with a step size of 0.017° and a counting time of 14 s per step. Qualitative phase analysis was conducted using the X’Pert Highscore plus search match software.
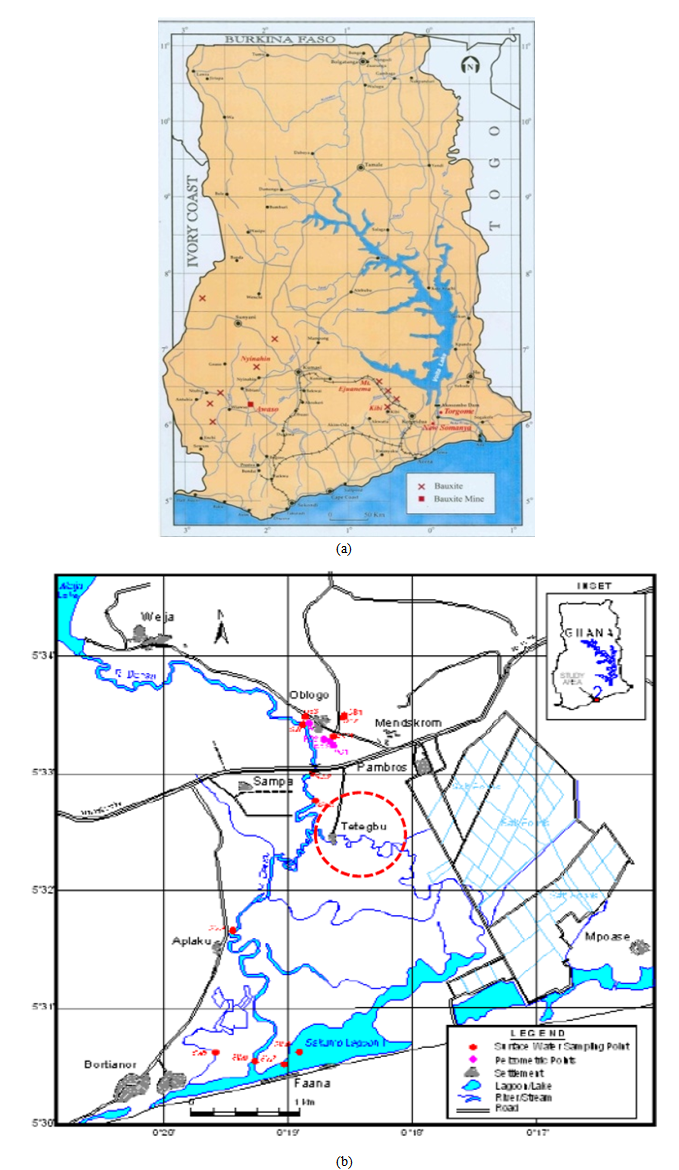 | Figure 2. Geological map of Ghana showing (a) bauxite deposits and (b) Tetegbu clay deposit site (red dash circle) |
|
 | Figure 3. Bauxite Red Mud-Clay briquettes |
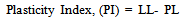 | (2) |
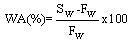 | (3) |
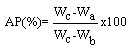 | (4) |
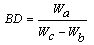 | (5) |
2.2. Flexural strength (Modulus of Rapture-MOR)
- Using three point bending testing (ASTM C99/C99M-09 standard protocols), the flexural strength of the test bars were determined. Figure-4 represent the test configuration for specimen dimension of (20 x 1 x 1) cm3 and distance 7.6 cm (between supports), monotonic loading was done at 1.85kg/min till point of fracture. Equation (6) was used in computing flexural strengths and the average values were recorded from two tests. The effect of clay content on the flexural strength values was investigated.
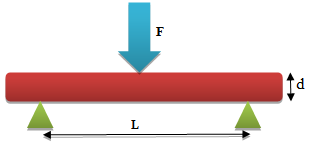 | Figure 4. Schematic Three-Point Bending Method |
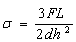 | (6) |
3. Results and Discussions
3.1. Chemical Analysis (X-Ray Florescence Spectroscopy)
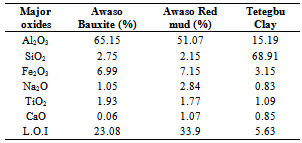
- The industrial applications of ceramic materials are greatly influenced by their mineralogical composition as well as their physical and chemical properties[20]. One of the widely used analytical tools in the determination of elemental/chemical composition of ceramic materials is the X-ray fluorescence spectroscopy. The XRF analytical results of the Awaso bauxite, its red mud (RM) and Tetegbu plastic clay are presented in Table-2 which agrees with results obtained by Liu et al.,[12].
|
3.2. XRD Analysis
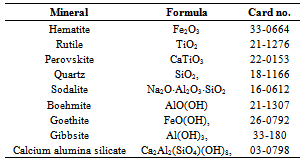
- The element analysis and phase characterization of clays, bauxite and red mud have been reported by various researchers[24,25] over the years with varying compositions of red mud This compositional difference is known to be dependent on the geographical location of the bauxite from which it was obtained.
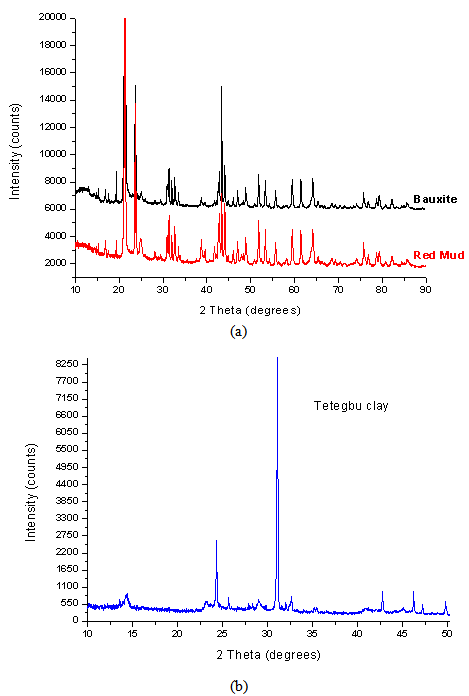 | Figure 5. XRD pattern of (a) Awaso Bauxite and Red Mud, and (b) Tetegbu Clay |
|
|
3.3. Thermal Analysis (TG-DTA)
- The main mineral phases of dried red mud at room temperature are calcite (CaCO3), dicalcium Silicate (Ca2SiO4), hematite (Fe2O3), perovskite (CaTiO3), gibbsite (Al(OH)3), and CaO. The TG-DTA thermograms (figure 6) show a continuous weight loss distributed in the range of 25–1200ºC. The figure shows two main portions of mass loss as the rise of temperature. The first one is during the heating temperature interval of 50–550◦C when the physically absorbed water and chemically bound water is off.
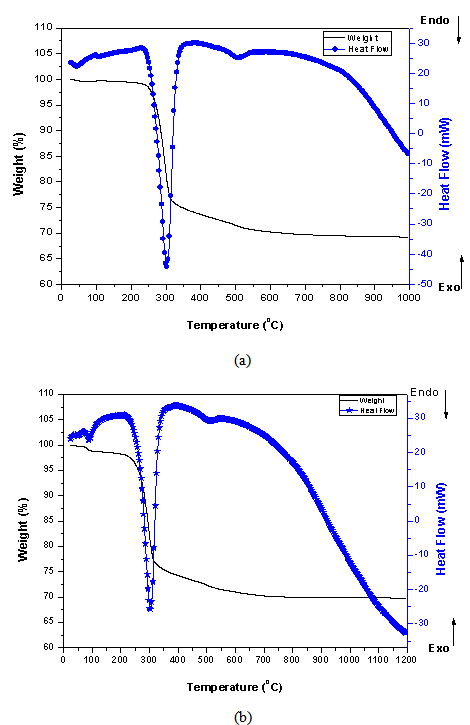 | Figure 6. Tg (Wt%)-DTA (Heat flow) thermographs of (a) Awaso Bauxite, and (b) Awaso Red mud |
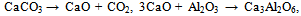 | (7) |
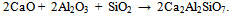 | (8) |
3.4. FTIR
- The molecular structure of red mud has been identified in the infrared region with Infrared Spectroscopy analysis. According to the given IR spectrum in Figure 7, the peak at 3426 cm-1 wavelength shows hydroxyl bonds of gibbsite. Free H2O peaks at 1642.88 and 1454.36 cm-1 wavelengths states CaO component in calcite.
 | Figure 7. FTIR of (a) Bauxite and (b) red mud samples |
3.5. Physical and Mechanical Properties
3.5.1. Plasticity Index (PI)
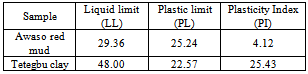
- In the building construction industry, determination of the plasticity index is very important, in ensuring that the sample (bricks or blocks) retains the correct amount of shear strength and not too much change in volume as it expands and shrinks at different moisture contents. Hence, the greater the plasticity index, the more plastic, compressible and greater the volume change characteristics of the ceramic samples.
|
3.5.2. Water of Absorption, Apparent Porosity, Bulk Density and Flexural Strength (Modulus of Rapture-MOR)
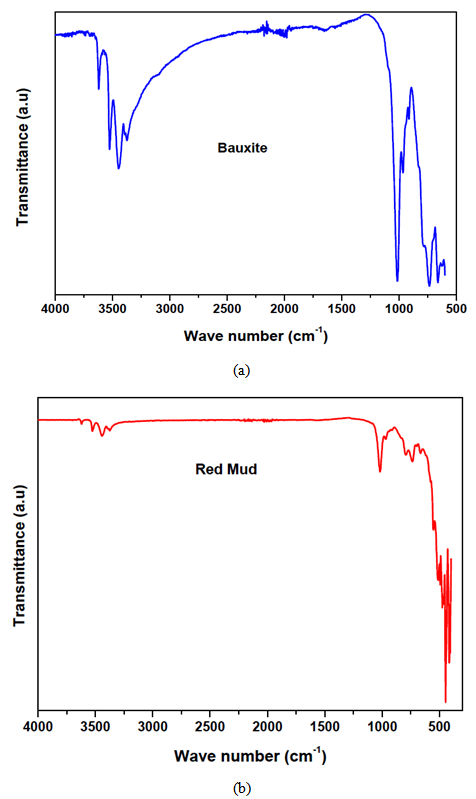
- During the firing/sintering of samples, there is a degree of linear shrinkage related to processes of pore formation and densification of the samples which influences the strength of the final test product. Increasing the red mud content tends to increase the bulk density, thereby decreasing their porosity and resulting in expansion of the samples. The presence of red mud leads to the formation of macropores which closes gradually as the quantity of red mud increases. The total porosity of the sintered samples can be open or closed. The porosity of the open-pore samples can be calculated from the results of water of absorption test. A low quantity of open pores is desirable; as it is an indication that the samples can better resist environmental conditions and possesses greater durability. It is worth stating that when there is a high degree of shrinkage of the brick samples, there will be a corresponding increase in the volume of the pores, thus lowering the absorption rate. The high content of the fluxes (see XRF, XRD and FTIR data) generates more vitreous phases which fill in the pores of the samples thereby decreasing the porosity and the quantity of water absorbed.The water of absorption of a test piece determines the measure of the extent to which the test piece is susceptible to seepage of water through its pores when immersed in water. This test gives an idea on how the brick products produced will behave when used in various applications. Test pieces having higher water of absorption means they are more porous in nature. It could also be observed that, as the clay content reduced, the water of absorption decreased. This could be attributed to the RM quantity in the fired brick and of all the batches. Low water of absorption in the test pieces will have higher strength and durability. Since if the water of absorption is low, it restricts the amount of water may cause deterioration. Water existing in the pores of the products will be cyclically expanded and contracted and this will generate stresses within the material thereby resulting in the weakening of the product.
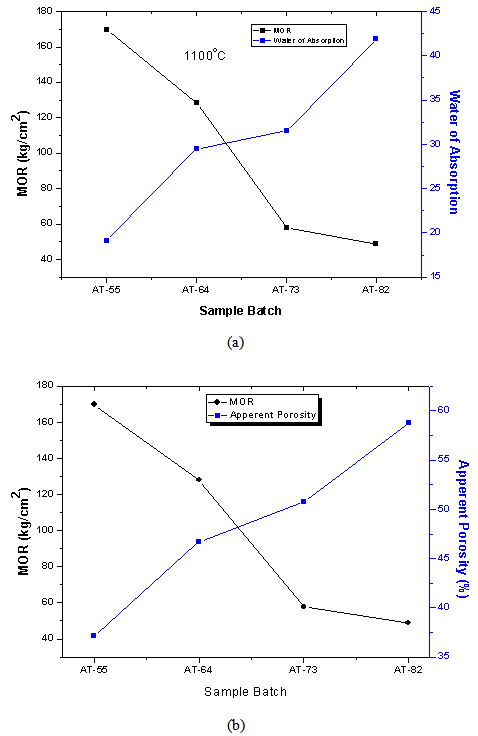 | Figure 8. variation of (a) water of absorption and (b) apparent porosity with modulus of rapture in relation to batch formulation of the composite bricks at 1100ºC |
3.5.3. Effect of Clay Content and Sintering Temperature on MOR
- Better sintering of the composite brick samples tend to increase with increase in temperature from 800 ºC and above leading to reduction in the average pore sizes. For the same sintering temperatures, the mechanical strength (modulus of rapture) was observed to increase with increasing composition of the Tetegbu clay as shown in Figure-9. This binder increment minimizes the porosity of the samples by increasing the cohesion forces while decreasing their inter-particles separation[26]. During the sintering process, polymorphic phase transformations occur as a result of chemical reactions leading into complex compounds at high temperatures.
3.6. Effect of Clay Content and Sintering Temperature on MOR
- Better sintering of the composite brick samples tend to increase with increase in temperature from 800 ºC and above leading to reduction in the average pore sizes. For the same sintering temperatures, the mechanical strength (modulus of rapture) was observed to increase with increasing composition of the Tetegbu clay as shown in Figure-9. This binder increment minimizes the porosity of the samples by increasing the cohesion forces while decreasing their inter-particles separation[26]. During the sintering process, polymorphic phase transformations occur as a result of chemical reactions leading into complex compounds at high temperatures. These tend to aid in the quick sintering process and impacts on the stability of the material due to the decrease or increase in the volume of the system[27, 28]. At temperatures beyond 900ºC, the presence of fluxes (K2O, Na2O, Fe2O3, and CaO) in the composite bricks begins to yield glassy phases aligning themselves very well along the grain boundaries of the mixtures to achieve high densification which increase the bulk density and consequently the mechanical strength.
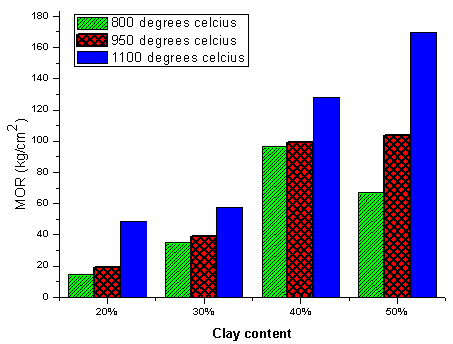 | Figure 9. Effect of Clay Content on Flexural Strength (MOR) at different firing temperatures |
4. Conclusions
- The possibility of using Bayer processed bauxite residue (red mud) as a raw material in brick production for building construction applications has been investigated. Bricks were fabricated from batch mixes of laboratory processed red mud and a highly plastic clay material, obtained locally in varying quantities. The chemical, physical and mechanical properties have been investigated for varying red mud-clay contents at firing temperatures of 800ºC, 950ºC, and 1100ºC. Sintering at 1100ºC produced the brick with the best properties mechanical properties. Physical properties such as apparent porosity and water of absorption reduced while the mechanical strength (modulus of rupture), and the bulk densities increased at higher sintering temperature. Considering the physical and mechanical properties of the fabricated brick samples, the batch formulation which contained 50% each of the Awaso red mud and Tetegbu clay is considered the best combination with optimal properties for the construction bricks application. Other batch formulations with 20-30% clay content were also within tolerable limits and could be employed in lighter weight structural applications.
ACKNOWLEDGEMENTS
- Authors would like to thank Mr. Emmanuel Frimpong. Brenya, Chief Technician at the Department of Materials Science and Engineering, University of Ghana for his immense contribution and Scientists at the Materials Institute of the Centre for Scientific and Industrial Research (CSIR), Ghana, are acknowledged for their technical assistance.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML