-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2013; 3(4): 63-69
doi:10.5923/j.materials.20130304.01
Ionic Liquids Applied to Improve the Dispersion of Calcium Oxide Nanoparticles in the Hydrogenated Acrylonitrile-Butadiene Elastomer
Magdalena Maciejewska, Alicja Krzywania-Kaliszewska, Marian Zaborski
Technical University of Lodz, Institute of Polymer and Dye Technology, 90-924 Lodz, Poland
Correspondence to: Magdalena Maciejewska, Technical University of Lodz, Institute of Polymer and Dye Technology, 90-924 Lodz, Poland.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Hydrogenated acrylonitrile-butadiene elastomer was cured with dicumyl peroxide in the presence of calcium oxide nanoparticles modified with 2,4-pentadienoic acid as a crosslinking co-agent and ionic liquids (alkylimidazolium salts) as a dispersing agent. In this article, we discuss the effect of the ionic liquids (alkylimidazolium salts) with respect to their anion (bromide, chloride, tetrafluoroborate, hexafluorophosphate) and the length of alkyl chain in the cation (allyl-, ethyl-, butyl-, hexyl-, octyl-) on the vulcanization kinetics of rubber compounds. The influence of ionic liquids on the cross-link density, the mechanical properties of the vulcanizates and their resistance to weather ageing were also studied. Alkylimidazolium salts improve the dispersion of the coagent particles and are active in the crosslinking of HNBR with peroxide. The application of ionic liquids increases the crosslink density of the vulcanizates and improves their resistance to weather aging.
Keywords: Calcium Oxide, Coagent, Ionic Liquid, Crosslinking, 2,4-Pentadienoic Acid
Cite this paper: Magdalena Maciejewska, Alicja Krzywania-Kaliszewska, Marian Zaborski, Ionic Liquids Applied to Improve the Dispersion of Calcium Oxide Nanoparticles in the Hydrogenated Acrylonitrile-Butadiene Elastomer, American Journal of Materials Science, Vol. 3 No. 4, 2013, pp. 63-69. doi: 10.5923/j.materials.20130304.01.
Article Outline
1. Introduction
- In recent years, significant attention has been focused on ionic liquids (ILs) due to their intrinsic properties, such as biocides[1]. The surface and interfacial properties are particularly important for the potential applications of ILs[2,3]. In addition, the synthesis of ionic liquids is not complex[4]. Ionic liquids can dissolve both organic and inorganic compounds as well as some polymers[4-7]. They can also be successfully used as accelerators for sulfur vulcanization of elastomers[8]. The preparation of new polymer composites and the improvement of their properties can also be achieved by using ionic liquids, because they may improve the dispersion, wettability and compatibility of the inorganic particles in an organic matrix[9]. Because ILs have very good ionic conductivity up to the decomposition temperature, they can play an important role in electrolyte matrixes[10-12].However, the vulcanizates crosslinked with peroxides have unsatisfactory mechanical properties for their To eliminate the disadvantages of peroxide vulcanization, coagents are used[14-18]. The application of coagents allows for noncovalent ionic or complex crosslinks due to the presence of functional groups in the coagent molecules that can form additional crosslinks[14,19,20]. In the literature, coagents that form hard domains during vulcanization surrounded by an elastomer with a lower stiffness have been investigated. Hard domains are created by coagents containing rigid aromatic structures. The domains with high rigidity partially contribute to the transfer of stresses in the elastomer network. The stress concentration near the hard coagent domains may deteriorate the physical properties of the vulcanizates[21]. Improvement in the mechanical properties can be achieved using coagents with more flexible structures to form soft domains of lower stiffness during vulcanization. These domains can deform under the influence of an external stress similar to the network formed by reinforcing filler particles[21,22]. The coagent’s activity in the crosslinking process is determined by the homogeneous dispersion of its particles in the elastomer matrix. When the dispersion of the coagent particles is more homogeneous, the area of their contact with the elastomer chains and the activity in crosslinking process are greater. Unfortunately, nanosized metal oxides agglomerate in the elastomer due to the nanoparticles’ high surface energy[23,24]. In addition, coagents based on mineral metal oxides may also act as fillers for the elastomers. Because the properties of the filler’s particles affect the structural properties of the composites, a suitable degree of filler dispersion in the elastomer matrix is required to afford effective reinforcement[25]. The difficulties in obtaining a uniform dispersion of coagent or filler in the elastomer contribute to the search for new substances to improve the dispersion of the nanoparticles. The application of ILs appears to be a good solution for this technological problem.The aim of this work was to improve the dispersion of a novel coagent, a nanosized calcium oxide modified with 2,4-pentadienoic acid in the HNBR elastomer crosslinked with dicumyl peroxide. The choice of ILs as dispersing agents was dictated by our earlier studies, which showed that alkylimidazolium salts improved the dispersion of zinc oxide nanoparticles in acrylonitrile-butadiene rubber[26]. Our studies focused on the effect of the ILs structure (cation and anion type) on the vulcanization kinetics of rubber compounds and the mechanical properties and the crosslink density of vulcanizates as well as their thermal stability and resistance to weather aging. The dispersion of coagent particles in the elastomer matrix was also examined.
2. Experimental Section
2.1. Materials
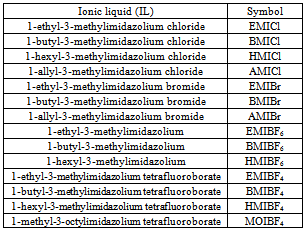
- Hydrogenated acrylonitrile-butadiene elastomer (HNBR, Therban A3407) containing 34 wt.% of acrylonitrile was obtained from Lanxess, Germany. Its Mooney viscosity was 70 at 100℃. The elastomer was cured with dicumyl peroxide (DCP, Aldrich). Nanosized calcium oxide (CaO, Aldrich) with a specific surface area of 50 m2/g (BET) was combined with an unsaturated carboxylic acid, such as 2,4-pentadienoic acid (PDA, Aldrich), and used as the crosslinking coagent. To improve the dispersion of the coagent’s particles in the HNBR elastomer, ionic liquids (Aldrich) were applied, and their characteristics are presented in Table 1.
|
2.2. Preparation and Characterization of Rubber Compounds
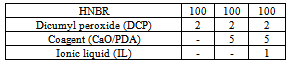
- Rubber compounds with the formulations given in Table 2 were prepared using a laboratory two-roll mill. The samples were cured at 160℃ until they developed a 90% increase in torque, which was measured using an oscillating disc rheometer (WG-02, Monsanto) according to ASTMD 2084-81.
|
2.3. Characterization of Vulcanizates
- The crosslink density (νT) of the vulcanizates was determined by their equilibrium swelling in toluene based on the Flory-Rehner equation[27]. The Huggins parameter of the elastomer-solvent interaction (χ) was calculated from the equation χ = 0.501 + 0.228Vr (Equation (1))[28], where Vr is the volume fraction of the elastomer in the swollen gel. To determine the content of ionic crosslinks in the elastomer network, samples were swollen in toluene in a desiccator with saturated ammonia vapor (25% aqueous solution). The content of ionic crosslinks (Δν) was calculated from Equation (2), where νA is the crosslink density determined for samples treated with ammonia vapor.
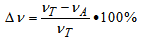 | (2) |
 | (3) |
3. Result and Discussion
3.1. Coagent Dispersion in the Elastomer
- As we previously reported, nanosized calcium oxide modified with unsaturated carboxylic acids had relatively large dispersive components of surface energy[24], which has a significant effect on their dispersion in the elastomer matrix. It may be expected that nanoparticles of CaO/PDA will agglomerate in the elastomer reducing the activity of coagent in the crosslinking process. To directly examine the dispersion of the coagent particles in the HNBR elastomer, SEM images of the vulcanizate surfaces were recorded. These results are presented in Figure 1 (a-d).CaO/PDA nanoparticles created agglomerates with complex morphologies that were several µm in size, which revealed poor adhesion to the elastomer matrix (Figure 1a). Such agglomerates may concentrate the stress in a sample subjected to external deformations initiating its premature destruction. Agglomerates also decrease the surface area of the coagent and as a result the surface of contact between coagent particles and elastomer chains. Therefore, the homogeneous dispersion of coagent particles is required to ensure the optimum activity of the coagent.The ILs improved the dispersion of CaO/PDA in the HNBR elastomer. The nanoparticles were homogeneously distributed in the elastomer matrix. For the BMIBr application, some aggregates were observed in the SEM image. However, their size was on the nanometer scale, and they exhibited good wettability by the elastomer.
 | Figure 1. SEM images of vulcanizates containing: a) CaO/PDA, b) BMICl, and c) BMICl |
3.2. Cure Characteristics and Crosslink Density of Vulcanizates
|
3.3. Mechanical Properties of Vulcanizates
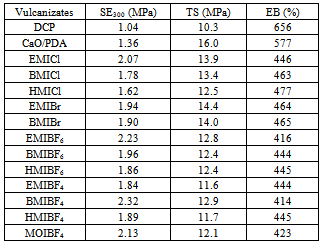
- The aim of coagent application is not only to improve crosslinking efficiency and the crosslink density of vulcanizates but also to increase their tensile strength. Therefore, the effect of CaO/PDA and ILs on the mechanical properties of vulcanizates was determined, and the results are provided in Table 5.
|
3.4. Thermal Stability of Vulcanizates
- Rubber products often work at elevated temperatures. If ILs are used as dispersing agents, they should not deteriorate the thermal stability of vulcanizates. Thermogravimetric analysis (TGA) was used to determine the thermal decomposition temperature of the HNBR vulcanizates and the total weight loss during decomposition. The results from these studies are presented in Table 6.
|
3.5. Vulcanizates Resistance to Weather Aging
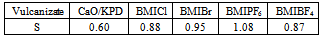
- Rubber products from HNBR elastomer during Rubber products from the HNBR elastomer are typically exposed to factors, such as elevated temperature, UV radiation, and humidity, that cause aging. Therefore, the effect of the ILs on the vulcanizates resistance to weather aging was examined via changes in the mechanical properties and the crosslink density of the vulcanizates (Figures 2-4).
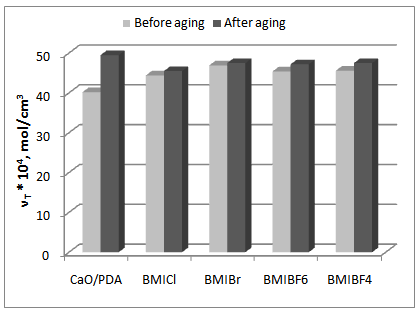 | Figure 2. Effect of weather aging on the crosslink density of vulcanizates |
 | Figure 3. Effect of weather aging on the elongation at break of vulcanizates |
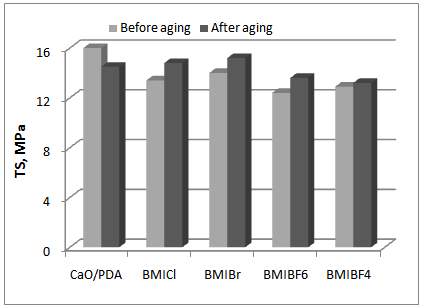 | Figure 4. Effect of weather aging on the tensile strength of vulcanizates |
|
4. Conclusions
- Nanosized calcium oxides modified with 2,4-pentadienoic acid reduced the vulcanization time of rubber compounds and increased the crosslink density and tensile strength of vulcanizates, which were due to the formation of labile, ionic crosslinks in the elastomer network. Therefore, CaO/PDA could be considered a suitable coagent for HNBR crosslinking with peroxides.CaO/PDA revealed a tendency to agglomerate in the elastomer, which decreased its activity in the crosslinking process. The application of ILs improved the dispersion of the coagent particles in the HNBR elastomer, reduced the vulcanization time, and increased the crosslink density of the vulcanizates. These results are mostly likely due to improved contact between the coagent particles and the elastomer chains resulting from its homogeneous dispersion as well as the catalytic effect of ILs on the interphase crosslinking reactions.The ILs did not improve the tensile strength of the vulcanizates. However, an increase in the thermal stability and resistance to weather was observed for HNBR.
ACKNOWLEDGEMENTS
- The authors wish to acknowledge the Polish Ministry of Science and Higher Education and the National Center for Research and Development for supporting this research.
References
| [1] | Pernak, J., Sobaszkiewicz, K., and Mirska, I., 2003, Anti-microbial activities of ionic liquids., Green Chemistry, 5, 52-56. |
| [2] | Materna, K., 2010, Surface activity of aqueous solutions of ionic liquids with saccharinate anion., Przemysl Chemiczny, 89, 1479-1482. |
| [3] | Kubisa, P., 2004, Application of ionic liquids as solvents for polymerization processes., Progress in Polymer Science, 29, 3-12. |
| [4] | Pernak, J., 2010, Ionic liquids as multifunctional compounds., Przemysl Chemiczny, 89, 1499-1503. |
| [5] | Wu. J., Zhang, J., Zhang, H., He, J.S., Ren, Q., and Guo, M., 2004, Homogeneous acetylation of cellulose in a new ionic liquid., Biomacromolecules, 5, 266-268. |
| [6] | Swatloski, R.P., Spear, S.K., Holbrey, J.D., and Rogers, R. D., 2002, Dissolution of cellulose with ionic liquids., Journal of American Chemical Society, 124, 4974-4975. |
| [7] | Biswas, A., Shogren, R.L., Stevenson, D.G., Willett, J.L., and Bhownik, P.K., 2006, Ionic liquids as solvents for biopolymers: Acylation of starch and zein protein. Carbohydrate Polymers, 66, 546-550. |
| [8] | Maciejewska, M., Zaborski, M., Kordala, R., and Walkiewicz, F., 2010, Ionic liquids as accelerators in elastomer vulcanization., Przemysl Chemiczny, 89, 1470-1474. |
| [9] | Kim, N.H., Malhotra, S.V., and Xanthos, M., 2006, Modifications of cationic nanoclays with ionic liquids., Microporous and Mesoporous Materials, 96, 29-35. |
| [10] | Yoshizawa, M., and Ohno, H., 2001, Synthesis of molten salt-type polymer brush and effect of brush structure on the ionic conductivity., Electrochimica Acta, 46, 1723-1728. |
| [11] | Yoshizawa, M., Ogihara, W., and Ohno, H., 2002, Novel polymer electrolytes prepared by copolymerization of ionic liquid monomers., Polymer Advanced Technolology, 13, 589-594. |
| [12] | Noda, A., and Watanabe, M., 2000, Highly conductive polymer electrolytes prepared by in situ polymerization of vinyl monomers in room temperature molten salts., Electrochimica Acta, 45, 1265-1270. |
| [13] | Dluzneski, P.R., 2001, Peroxide vulcanization of elastomers., Rubber Chemistry and Technology, 74, 451-492. |
| [14] | Dikland, H.G., van der Does, and L., Bantjes, A., 1993, FT-IR spectroscopy, a major tool for the analysis of peroxide vulcanization processes in the presence of coagents. I: Mechanism of EPM peroxide vulcanization with aromatic bis(allyl)esters as coagents., Rubber Chemistry and Technology, 66, 196-212. |
| [15] | Garcia-Quesada, J.C., and Gilbert, M., 2000, Peroxide crosslinking of unplasticized poly(vinyl chloride)., Journal of Applied Polymer Science, 77, 2657-2666. |
| [16] | Costin, R., and Henning, S.K., 2006, Fundamentals of curing elastomers with peroxides and coagents., Rubber World, 233, 28-35. |
| [17] | De Risi, F. R., and Noordermeer, J. W. M., 2007, Effect of me-thacrylate co-agents on peroxide cured PP/EPDM thermoplastic vulcanizates., Rubber Chemistry and Technology, 80, 83-99. |
| [18] | Oh, S.J., and Koenig, J.L., 1999, Studies of peroxide curing of cis-1,4-polyisoprene/diallyl phthalate blends byspectroscopic techniques., Rubber Chemistry and Technology, 72, 334-342. |
| [19] | Nie, Y., Huang, G., Qu, L., Zhang, P., Weng, G., and Wu, J., 2010, Cure kinetics and morphology of natural rubber reinforced by the in situ polymerization of zinc dimethacrylate., Journal of Applied Polymer Science, 115, 99-106. |
| [20] | Lu, Y., Liu, L., Tian, M., Geng, H., and Zhang, L., 2005, Study on mechanical properties of elastomers reinforced by zinc dimethacrylate., European Polymer Journal, 41, 589-598. |
| [21] | Keller, R.C., 1988, Peroxide curing of ethylene-propylene elastomers., Rubber Chemistry and Technology, 61, 238-254. |
| [22] | Drake, R.E., Holliday, J.J., and Costello, M.S., 1995, Use of polybutadiene coagents in peroxide cured elastomers for wire and cable., Rubber World, 213 (3), 22-30. |
| [23] | Przybyszewska, M., Krzywania, A., Zaborski, M., and Szynkowska, M. I., 2009, Surface properties of zinc oxide nanoparticles studied by inverse gas chromatography., Journal of Chromatography A, 1216, 5284-5291. |
| [24] | Maciejewska, M., Krzywania-Kaliszewska, A., and Zaborski, M., 2012, Surface properties of calcium and magnesium oxide nanopowders grafted with unsaturated carboxylic acids studied with inverse gas chromatography., Journal of Chromatography A.,1257, 141-148. |
| [25] | Bieliński, M.D., Dobrowolski, O., and Ślusarski, L., 2007, Dispersion of a filler in the rubber blend. Part 1. Dispergation process description., Polimery, 52, 546-555. |
| [26] | Przybyszewska, M., and Zaborski, M., 2010, Effect of ionic liquids and surfactants on zinc oxide nanoparticle activity in crosslinking of acrylonitrile butadiene elastomer., Journal of Applied Polymer Science, 116, 155-164. |
| [27] | Flory, P. J., and Rehner, J., 1943, Statistical mechanics of cross-linked polymer networks. II. Swelling., Journal of Chemical Physics, 11, 521-526. |
| [28] | Przybyszewska, M., and Zaborski, M., 2009, New coagents in crosslinking of hydrogenated butadiene-acrylonitrile elastomer based on nanostructured zinc oxide., Composite Interfaces, 16, 131-141. |
| [29] | Masek, A., Zaborski, M., and Kosmalska, A., 2011, Derivatives of flavonoides as anti-ageing substances in elastomers., Comptes Rendus Chimie, 14, 483-488. |
| [30] | Lu, J., Yan, F., and Texter, J., 2009, Advanced applications of ionic liquids in polymer science., Progress in Polymer Science, 34, 431-448. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML