-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2013; 3(3): 55-61
doi:10.5923/j.materials.20130303.02
A Comparison Study of Liquid Natural Rubber (LNR) and Liquid Epoxidized Natural Rubber (LENR) as the Toughening Agent for Epoxy
S. K. Tan1, S. Ahmad1, C. H. Chia1, A. Mamun2, H. P. Heim2
1School of Applied Physics, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, UKM Bangi, 43600, Malaysia
2Institut für Werkstofftechnik, University of Kassel, Mönchebergstrasse-3, Kassel, 34125, Germany
Correspondence to: S. K. Tan, School of Applied Physics, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, UKM Bangi, 43600, Malaysia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Rubber-toughened epoxy resins were prepared using the mechanical stirring method and molded into samples by compression molding. The aim of this study is to modify the brittleness of the epoxy matrix by adding discrete rubbery phases to improve the toughness properties. Liquid natural rubber (LNR) and liquid epoxidized natural rubber (LENR) were used as toughening agents in the epoxy resin to compare the properties of the modified networks. The mechanical and thermal properties have been studied to observe the effect of the modified epoxy network. It was found that by adding the rubbery phase into epoxy resin, the toughness of the epoxy was improved. A composite with 3 wt% of LENR possessed the highest mechanical properties for both flexural and impact properties. The scanning electron micrograph (SEM) demonstrated the discrete rubbery phases between the epoxy and the rubber particles. The glass transition temperature shifted to a lower temperature in the dynamic mechanical analysis (DMA) for the rubber-toughened epoxy.
Keywords: Liquid Natural Rubber, Liquid Epoxidized Natural Rubber, Epoxy, Rubber-toughened Epoxy, Toughness, Flexural Test, Impact Test, Scanning Electron Micrograph, Dynamic Mechanical Analysis
Cite this paper: S. K. Tan, S. Ahmad, C. H. Chia, A. Mamun, H. P. Heim, A Comparison Study of Liquid Natural Rubber (LNR) and Liquid Epoxidized Natural Rubber (LENR) as the Toughening Agent for Epoxy, American Journal of Materials Science, Vol. 3 No. 3, 2013, pp. 55-61. doi: 10.5923/j.materials.20130303.02.
Article Outline
1. Introduction
- Epoxy resins are considered one of the most important classes of thermosetting polymers. With their wide-ranging high-performance, epoxy resins are extensively used in many applications including high performance adhesives, aerospace, automobiles and for other engineering purposes [1]. The most widely used epoxy is epichlorohydrin and bisphenol-A derived resins. The outstanding performance characteristics of these resins are conveyed by the bisphenol-A, ether linkages and the hydroxyl and epoxy groups. Bisphenol-A gives the toughness, rigidity and maintains the properties of epoxy in elevated temperatures. The ether linkages provide chemical resistance. The hydroxyl and epoxy groups provide adhesive properties and formulation latitude, or reactivity with a wide variety of chemical curing agents. Once epoxy cured, they are characterized by high chemical and corrosion resistance as well as good mechanical and thermal properties. However, the chief drawbacks of epoxy from industrial use are their brittleness and high cost[2]. Therefore, modifying epoxy resins has attracted intense research interest. When it comes to modifying the brittle nature of epoxy, an increase in toughness without any reduction in the other important properties is required, such as modulus, thermal properties and environmental corrosion resistance. One of the methods used to improve the toughness of epoxy involves the addition of a rubbery content into the uncured epoxy resins[3]. The rubbery materials that are added to the uncured epoxy are types of copolymers with variable acrylonitrile contents. Studies reported to modify epoxy resin were mostly modified liquid rubber, such as liquid rubber modified by divinylbenzene (DVB), hydroxyl terminated butadiene (HTPB), carboxyl terminated butadiene-acrylonitrile (CTBN), or isocyanate terminated polybutadiene (NCOPBER)[2, 4-6]. The particle size, concentration of liquid rubber, dispersion of liquid rubber in the epoxy matrix, curing and interaction between rubber and epoxy have been taken into consideration and studied to improve the impact strength and the modulus. According to Riew and Smith (1989), the first article concerning rubber as the toughening agent in polymer was published in 1956 by Merz et al., which addressed a rubber-toughening mechanism for a high impact polystyrene (HIPS) system. Since then, many researchers have studied the mechanism of rubber as the toughening agent in plastics[7]. Phinyocheep et al. (2007) discussed poly (ethylene terephthalate) toughened by natural rubber. The impact strength increased by increasing the rubbery phase which is well dispersed in the matrix phase[8]. Therefore, the dispersion of the rubber particles in the matrix is one of the main factors that affect the mechanical properties of the material. In this report, liquid natural rubber (LNR) and liquid epoxidized natural rubber (LENR) were used as the toughening agent for the epoxy matrix. Based on the theory of rubber as the toughening agent for the matrix, a small amount of rubber particles dispersed in the matrix can have a distinct influence on the final mechanical behavior of the matrix. At first, the natural rubber (NR) used is an elastomer, which is derived from plants and consists mostly of cis–1,4–polyisoprene with a repeating hydrocarbon unit, (-CH2CH3C=CHCH2-)[9]. Naturally, NR possesses some abnormal groups, such as epoxide, amine and hydroxyl functions, as reported in the literature. NR shows very interesting physical properties due to its ability to crystallize under stretching. However, it has a low elasticity because the polymer chains are only interlinked at a few points. To increase the elasticity and strength of NR, vulcanization, a curing process involving the addition of sulfur and high heat to create sulfur cross linking, is commonly employed[10, 11].
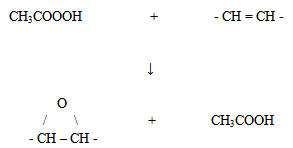 | Figure 1. The epoxidation reaction of natural rubber |
2. Experimental
2.1. Materials and Sample Preparation
- Epoxy resin with the grade of Epikote 828, and Jeffamine polyoxypropylenediamine D230 were purchased from Asachem (M) Sdn. Bhd. Epoxidized natural rubber with grade ENR-50 and natural rubber were obtained from the Rubber Research Institute Malaysia (RRIM). Liquid natural rubber (LNR) and liquid epoxidized natural rubber (LENR) were prepared by the photosynthesized degradation of the ENR in visible light according to a method described by Abdullah and Ahmad (1992)[15]. A mechanical stirrer was used in this research to mix the materials. First, the LNR or LENR and epoxy were stirred for 1 hour at a speed of 1000 rpm. After 1 hour, the curing agent was added and the mixture was stirred for a further 15 minutes. Finally, the sample was pressed in a mold using compression molding for 15 minutes at 110ºC, with 4 minutes preheating. The pressed sample was put into an oven to post cure for 2 hours at 80ºC. Samples were prepared with different rubber compositions in the matrix. The neat epoxy was prepared as the control.
2.2. Sample Characterization
- Flexural properties were measured under a three-point-bending approach using a Testometric M350-10CT according to ASTM D790. The size of the specimen was 127 by 12.7 by 3.2 m, which was tested flatwise on a support span, resulting in a support span-to-depth ratio of 16. Flexural properties were measured using a 1000 N load cell and a cross head speed of 1.37 mm/min. An impact test was performed using a Ray Ran RR/IMT digital universal impact tester according to ASTM D256. The size of the specimen flatwise before the notch was 63.5 by 12.7 by 3.2 mm. The width of the specimen was the thickness of the sheet. The depth of the plastic material remaining in the specimen under the notch was 10.16 ± 0.05mm. The fractured surfaces of the samples were coated with a gold layer and examined using scanning electron microscopy, SEM (Philips XL-30). Dynamic mechanical analysis (DMA) was evaluated by using a Universal V4.2E TA machine with a single-cantilever mode. Samples were tested at a heating rate of 5ºC/min and a frequency of 1 Hz from room temperature to 140ºC.
3. Results and Discussion
3.1. Flexural Properties
- Figure 2 and Figure 3 show the comparison of the bending properties of rubber-toughened epoxy with neat epoxy.
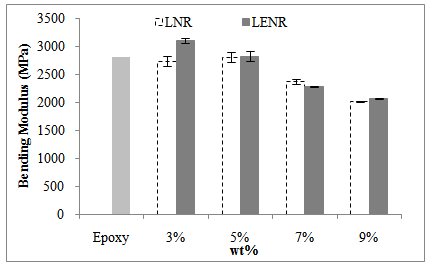 | Figure 2. The comparison of bending strength of rubber-toughened epoxy with neat epoxy |
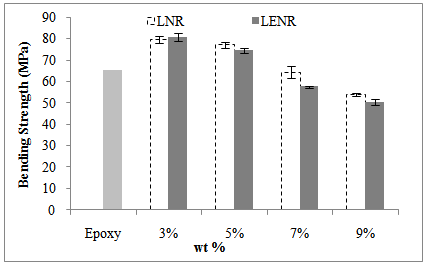 | Figure 3. The comparison of bending modulus of rubber-toughened epoxy with neat epoxy |
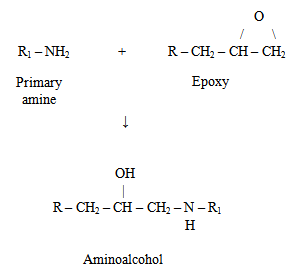 | Figure 4. The reaction of primary amine with epoxy |
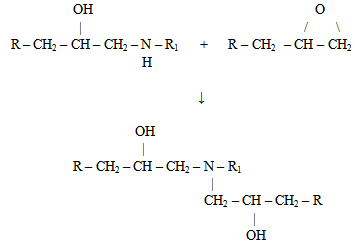 | Figure 5. The reaction of “secondary” amine with another epoxy or oxirane group |
3.2. Impact Properties
- Samples that were used in this testing were all notched samples. The notches in the izod impact specimen serve to concentrate the stress, minimize plastic deformation, and direct the fracture to the part of the specimen behind the notch[4]. In a notched sample, an apparent crack was created; therefore, the amount of energy absorbed depends primarily on the energy to propagate the crack. Figure 6 shows the comparison of impact strength of rubber-toughened epoxy with neat epoxy. When LNR or LENR was added to the epoxy system, the impact strength for both series of samples increased. For the series of LNR toughened epoxy, 5 wt% of LNR toughened epoxy achieved the optimum results. Whilst for the LENR series, when 3 wt% LENR was added to the epoxy resin, it showed the optimum result. However, the impact strength decreased gradually as the LENR content increased. At the same time, 3 wt% of LENR toughened epoxy achieved the highest impact strength in this study. From the impact test, we see that using the rubbery phase as a toughening agent to the epoxy matrix has a beneficial effect. Adding the discrete rubbery phase could improve the impact strength of the epoxy.
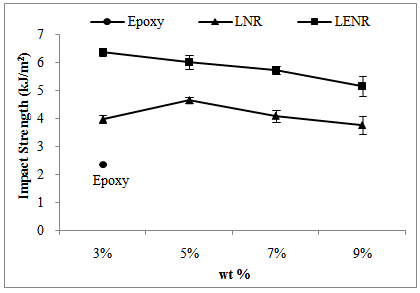 | Figure 6. The comparison of impact strength of rubber-toughened epoxy with neat epoxy |
3.3. Morphological Analysis
- Rubbers are generally well known for affecting the modulus of a matrix depending on their compatibility with the matrix, the surface area in contact, particle size and the shape of the rubber existing in the matrix, as well as the intrinsic strength of the rubbery phase in the matrix[5]. The impact behavior of the toughened networks can be explained by considering both the toughening and flexibility effects. The flexibility effect is caused by the presence of dissolved rubber inside the epoxy matrix, whereas the toughening process is related to the cavitations in the rubber particles dispersed inside the epoxy matrix[4]. In order to correlate the mechanical properties with the morphological analysis, the particle size and the fracture surfaces of the rubber-toughened epoxy were analyzed by the scanning electron microscope (SEM). Figure 7 shows the morphological analysis of the neat epoxy and rubber toughened epoxy at a magnification of 1000×, showing the differences in the particle size of the samples.Figure 7 (a) is the fracture surface of the neat epoxy. It can be observed that a few voids exist in the SEM micrograph. The existing voids are an unavoidable issue in the epoxy matrix. These voids could act as the stress concentrators bringing about the brittleness of the cured epoxy. Figures 7 (b) and (c) are SEM micrographs of the fracture surfaces of 3 wt% and 7 wt% of LENR toughened epoxy. Heterogeneous morphology surfaces resulted on the fracture surfaces. The holes observed in the micrograph indicate that the rubber particles dispersed in the epoxy matrix. The cavitations show that the addition of liquid rubber acts as the toughening agent to the epoxy matrix. Figures 7 (b) and (c) also show that there are differences in the particle size of the LENR dispersed in the epoxy matrix. The particle sizes were measured during SEM analysis, and the average results were obtained. The sample with 3 wt% LENR toughened epoxy showed a particle size of about 0.5 to 1.0 µm, whilst the sample with 7 wt% LENR toughened epoxy showed particle size to be about 1.0 to 1.5 µm. This shows that the particle sizes of rubber in the epoxy matrix increased as the LENR content increased. Figures 4 (d) and (e) show the SEM micrograph of 5 wt% and 9 wt% LNR toughened epoxy, respectively. The holes present in the micrograph indicate the LNR rubber particles. In Figure 7 (d), the particle size is about 7.5 to 10 µm, whilst in Figure 7 (e) the particle size is about 5 to 25 µm and is non-uniform. The presence of larger particle sizes is attributed to the agglomeration of the rubber particles with the increase of the rubber content in the epoxy matrix[4, 13]. From the SEM micrographs, the rubber phase appears as a spherical particle in the epoxy matrix. The spherical rubber domain is usually observed in the polymerization induced spinodal decomposition of the rubber modified epoxy systems. The formation of the rubber phase is generally attributed to the spinodal decomposition caused by the increase in the molecular weight of the epoxy matrix when the samples were curing. The phase separation starting from an initial rubber/epoxy co-continuous structure and the influence of surface tension in the separated phase usually results in a spherical rubber particle during cure[18].
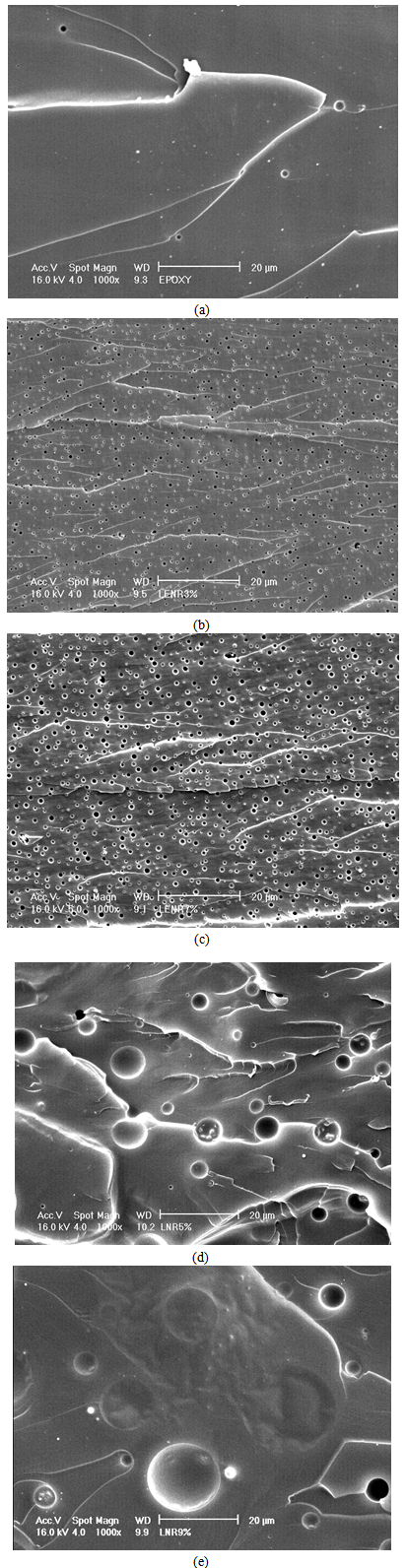 | Figure 7. SEM micrographs of the fracture surface for (a) neat epoxy, (b) 3 wt% LENR, (c) 7 wt% LENR, (d) 5 wt% LNR, and (e) 9 wt% LNR toughened epoxy, at the same magnification of 1000× |
3.4. Dynamic Mechanical Analaysis (DMA)
- Figure 8 presents the temperature dependence graph of (a) storage modulus, and (b) tan delta of the LNR toughened epoxy. Whilst Figure 9 shows the temperature dependence of (a) storage modulus, and (b) tan delta of the LENR toughened epoxy.
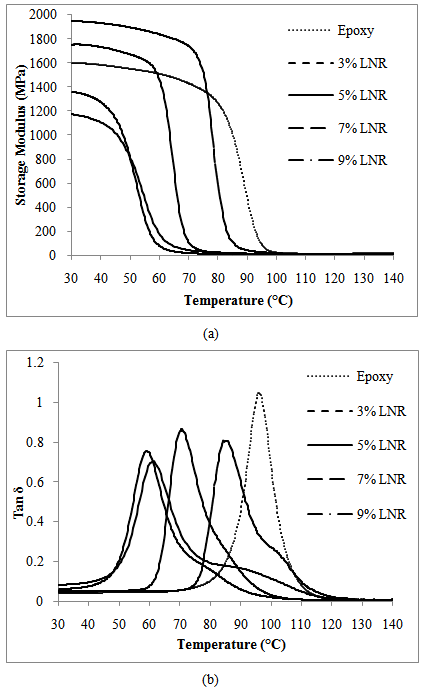 | Figure 8. Temperature dependence of (a) Storage modulus, and (b) Tan delta of LNR toughened epoxy |
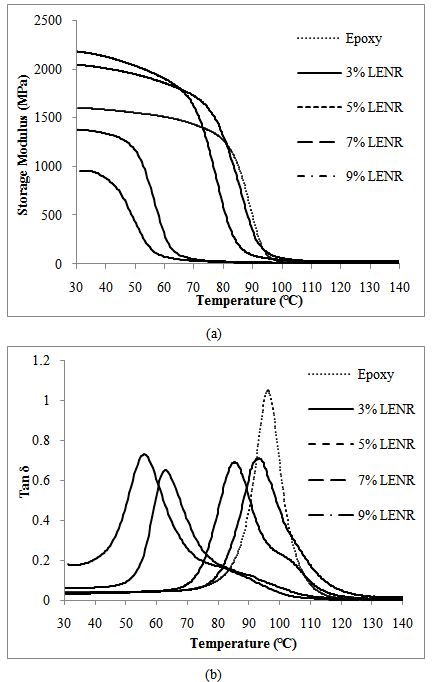 | Figure 9. Temperature dependence of (a) Storage modulus, and (b) Tan delta of LENR toughened epoxy |
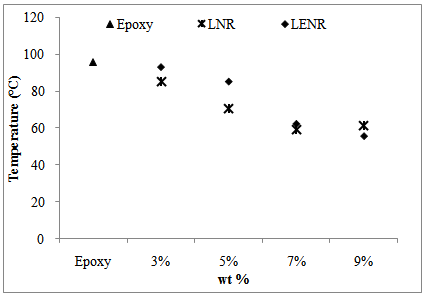 | Figure 10. The comparison of glass transition temperature of rubber-toughened epoxy |
4. Conclusions
- The results show that liquid epoxidized natural rubber is a good potential toughening agent for epoxy resin. With the addition of liquid rubber to the epoxy matrix, a significant increase in the bending properties and impact strength was observed. The sample with 3 wt% of LENR toughened epoxy obtained optimum results for both bending and impact properties. The heterogeneous morphology of the fracture surfaces was obtained by SEM analysis and shows that different particle sizes exist in the rubber-toughened epoxies. From the SEM micrographs, the LENR toughened epoxy had better compatibility with the epoxy resin as the particle size dispersed in the epoxy matrix was smaller and distributed more evenly than LNR. The addition of rubber content to epoxy resin decreased the glass transition temperature. The lower content of rubber in epoxy resin increased the storage modulus, which led to the improvement in the toughness of the epoxy. Overall, LENR as a toughening agent to epoxy resins possessed better mechanical and thermal properties compared to the LNR toughened epoxy. This is because the LENR had better compatibility with the epoxy network.
ACKNOWLEDGEMENTS
- The author (S.K. Tan) is thankful to the National Science fellowship of Malaysia for the financial support to the author during her study for this research.
References
| [1] | Mathew, V. S., Sinturel, C., George, S. C., Thomas, S., Epoxy resin/ liquid natural rubber system: secondary phase separation and its impact on mechanical properties, Journal of Materials Science, 45, 1769-1781 (2010) |
| [2] | Thomas, R., Ding, Y., He, Y., Yang, L., Moldenaers, P., Yang, W., Czigany, T., Thomas, S., Miscibility, morphology, thermal, and mechanical properties of a DGEBA based epoxy resin toughened with liquid rubber, Polymer, 49, 278-294 (2008) |
| [3] | Hsieh, T. H., Kinloch, A. J., Masania, K., Lee, J. S., Taylor, A. C., Sprenger S., The toughness of epoxy polymers and fibre composites modified with rubber microparticles and silica nanoparticles, Journal of Materials Science, 45, 1193-1210 (2010) |
| [4] | Barcia, F. L., Amaral, T. P., Soares, B. G., Synthesis and properties of epoxy resin modified with epoxy-terminated liquid polybutadiene, Polymer, Vol. 44, Issue 19, 5811-5819 (2003) |
| [5] | Saadati, P., Baharvand, H., Rahimi, A., Morshedian, J., Effect of Modified Liquid Rubber on Increasing Toughness of Epoxy Resins, Iranian Polymer, 14, 7, 637-646 (2005) |
| [6] | Szeluga, U., Kurzeja, L., Galina, H., Curing of epoxy/novolac system modified with reactive liquid rubber and carbon filler, Polymer bulletin, 60, 555-567 (2008) |
| [7] | Riew, C. K., Smith, R. W., Rubber-toughened plastics, American chemical society (1989) |
| [8] | Phinyocheep, P., Saelao, J., Buzaré, J. Y., Mechanical properties, morphology and molecular characteristics of poly(ethylene terephthalate) toughened by natural rubber, Polymer, 48, 5702-5712 (2007) |
| [9] | Zhang, C., Wang, W., Huang, Y., Pan, Y., Jiang, L., Dan, Y., Luo, Y., Peng, Z., Thermal, mechanical and rheological properties of polylactide toughened by epoxidized natural rubber, Materials and Design, 45, 198-205 (2013) |
| [10] | Arroyo, M., Lo′pez-Manchado, M. A., Valentı′n, J. L., Carretero, J., Morphology/behaviour relationship of nanocomposites based on natural rubber/epoxidized natural rubber blends, Composites Science and Technology, 67, 1330–1339 (2007) |
| [11] | Klysubun, W., Thanawan, S., Thamasirianunt, P., Radabutra, S., Sombunchoo, P., Determination of chlorine content in chlorinated, vulcanized natural rubber by XANES, PII, Vol. 582, 242-244 (2007) |
| [12] | Bussi, P., Ishida, H., Partially miscible blends of epoxy resin and epoxidized rubber: structural characterization of the epoxidized rubber and mechanical properties of the blends, Journal of Applied Polymer Science, 53, 441-454 (1994) |
| [13] | Gan, S. N., Abdul, H. Z., Partial conversion of epoxide groups to diols in epoxidized natural rubber, Polymer, Vol. 38, 8, 1953-1956 (1996) |
| [14] | Yu, H., Zeng, Z., Lu, G., Wang, Q., Processing characteristics and thermal stabilities of gel and sol of epoxidized natural rubber, European Polymer Journal, 44, 453-464 (2008) |
| [15] | Abdullah, I., Ahmad, S., Liquid natural rubber as a compatibiliser in the blending of natural rubber with polypropylene, Material Forum, 16, 353-357 (1992) |
| [16] | Jeffamine Polyoxypropyleneamine Curing Agents for Epoxy Resins. Texas: Texaco Chemical Company. (1993) |
| [17] | Bucknall, C. B., Smith, R. R., Stress whitening in high-impact polystyrene, Polymer, 6, 437 (1965) |
| [18] | Hong, S. G., Chan, C. K., The curing behaviors of the epoxy/dicyanamide system modified with epoxidized natural rubber, Thermochimica Acta, 417, 99-106 (2004) |
| [19] | Thomas, R., Durix, S., Sinturel, C., Omonov, T., Goosens, S., Groeninckx, G., Moldenaers, P., Thomas, S., Cure kinetics, morphology and miscibility of modified DGEBE-based epoxy resin – Effects of liquid rubber inclusion, Polymer, 48, 1695-1710 (2007) |
| [20] | Pire, M., Norvez, S., Iliopoulos, I., Rossignol, B. L., Leibler, L., Imidazole-promoted acceleration of crosslinking in epoxidized natural rubber/dicarboxylic acid blends, Polymer, 52, 5243-5249 (2011) |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML