-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2013; 3(1): 19-23
doi:10.5923/j.materials.20130301.03
Understanding Interactions between Cellulose and Phosphate Esters in Papermaking
Elhadji Babacar Ly1, 2, François Brouillette1
1Lignocellulosic Materials Research Center (CRML), Université du Québec à Trois-Rivières, Trois-Rivières, G9A 5H7, Canada
2Département de Chimie, Université de Ziguinchor, Ziguinchor, BP523, Senegal
Correspondence to: Elhadji Babacar Ly, Lignocellulosic Materials Research Center (CRML), Université du Québec à Trois-Rivières, Trois-Rivières, G9A 5H7, Canada.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Linting is a major problem affecting printing processes. It is characterised by the detachment of fibres from paper surface and their accumulation on press blankets. Recently, the addition of blends of phosphate esters to the pulp suspension prior to sheet formation has been identified as an effective way of reducing the linting propensity of paper. The objective of this study was to understand how phosphate esters are retained on cellulosic substrates and how they can contribute to the potential reduction of the linting of paper. Techniques like XPS, MS and conductometric titration were used to give an explanation of this phenomenon. The results tend to confirm the presence of phosphorus on cellulosic substrates and provide evidence of phosphate ester-cellulose linkages. This study allows us to elucidate part of the retention mechanism of phosphate esters in paper and explain their lint reduction ability.
Keywords: Cellulose, Phosphate Esters, Linting, Papermaking
Cite this paper: Elhadji Babacar Ly, François Brouillette, Understanding Interactions between Cellulose and Phosphate Esters in Papermaking, American Journal of Materials Science, Vol. 3 No. 1, 2013, pp. 19-23. doi: 10.5923/j.materials.20130301.03.
Article Outline
1. Introduction
- Nowadays, chemical products are commonly added to the wet pulp to improve paper properties[1-5]. Among these many chemicals, phosphate esters have been shown to reduce the linting propensity of paper during the printing process.However, this effect was mainly demonstrated by industrial experience[2-4]. The exact mechanism by which phosphate esters prevents linting is still under investigation. It has been proposed that the surfactant nature of phosphate esters made then act as “internal” release agents preventing the partial detachment of fibres during sheet pressing and drying thus reducing the complete detachment of fibres in the printing process (linting)[5]. This paper is an attempt to determine if phosphate esters are retained in the sheet during formation on the papermachine and, if it is the case, by what kind of interaction with the fibres. However, it was shown that the efficiency of these molecules depend greatly on variables such as product solubility, alkyl chain length and type, physicochemical parameters of the pulp suspension (pH, calcium ion concentration) and adsorption on fibres. Therefore, the aim of this study was to investigate the occurrence of phosphate esters action in lint reduction applications by trying to find interactions between cellulose fibres and phosphate esters.
2. Experimental
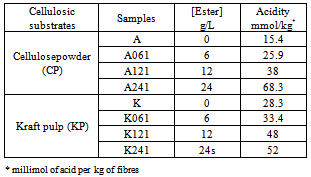
- In this investigation, cellulose powder (CP, 20 µm, Aldrich) and bleached kraft pulp (KP, Kruger Wayagamack Inc., Trois-Rivières Canada) were suspended in solutions of phosphate esters in standard papermaking conditions. Phosphate esters consisted in a commercial blend of C8 to C12 mono- and di- esters. Its exact component was already determined[6].
2.1. Methods
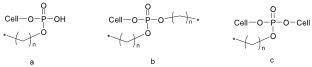 | Figure 1. Possible cellulose-Phosphate ester reaction products |
2.2. Physicochemical Characterisation
- Several analytical techniques like mass spectrometry (MS), conductometric analysis and X-ray photoelectron spectroscopy (XPS) were used to characterise treated fibres and recovered solutions. The composition of recovered solutions was determined by MICROMASS spectrometer (QUATROMICRO model). Samples were injected by syringe and ionized by the electrospray method (ESI).Conductometric titrations were performed with a THERMO ORION conductometer (model 150). The titration of acidic groups required a pre-treatment of dried fibres. Therefore, 6g of dried fibres were treated with 200 mL of 0.1N HCl for 45 min, filtered and washed 5 times with 100 mL of demineralised water. This step was repeated once again. The titration was performed by suspending, 3g of pre-treated fibres in 400 mL of 0.001N NaCl and 5 ml of 0.100N HCl. The suspension was held at room temperature and stirred under nitrogen gas flow to minimise carbon dioxide interference. A 0.0100N NaOH solution was added at a rate of 0.1 mL/min with continuous stirring[7-9].XPS experiments were recorded on a KRATOS ULTRA ELECTRON SPECTROMETER (Kratos Analytical) using a monochromatic Al Kα X-ray source (15 kV, 5 mA). The samples were previously dried for a week and then placed in an ultrahigh vacuum chamber (10-8 mbar) and collected data were analysed by CASA XPS software. The C-H signal was used as a reference peak at 284.9 eV. A complete description of the XPS characterisation method can be found in the literature[10].
3. Results and Discussion
3.1. Recovered Solution Analysis
- In agreement with Daemen results[6], the presence of different hydrogeno-phosphate (mono- and di-ester) was observed in the original ester blend (Z) (Table 1). After 6h of contact with cellulosic substrates and filtration, monohydrogen phosphates, identified mC8 and mC10, were not detected in the recovered solution indicating a reaction with cellulose fibres or an adsorption on their surface (Table 1). Moreover, MS results also showed that all phosphate esters in the mixture did not react equally with fibres. This is quite normal, because monohydrogen phosphates are supposed to have a higher reactivity than dihydrogen.In the original ester mixture, the relative amounts of mC8 and mC10 are 9.3% and 6.3% respectively. After 6h of reaction with cellulose, at least 76% and 100% of monohydrogen phosphate reacted with 6g/L and 12g/L of mixture solution. We can also observe in Table 1 that the proportion of monohydrogen phosphate decreased with increasing concentration of ester solution. Similar results were obtained for the two studied cellulosic substrates. After 6h of treatment, two phases were obtained. The reduction of the quantity of monohydrogen phosphate in recovered solutions tends to indicate that hydrogen phosphates are adsorbed onto cellulose substrates.The LC-MS results show that there are hydrogen phosphates adsorbed onto cellulosic substrates.After6 hours of treatment,two phases were obtained (liquid and solid).Since hydrogen phosphates were not found in the liquid phase, they should all be in the solid phase (fibres). But in light of these results, some reflections must be taken into account. Probably some dihydrogen phosphate may be adsorbed on the fibres without chemical bonds.
3.2. Fibres Characterization
- Results of conductometric titration of acidic groups for CP and KP fibres are presented in Table 2. Titration curves of treated fibres exhibit a plateau and a shift of the equivalence point (Figure 2).
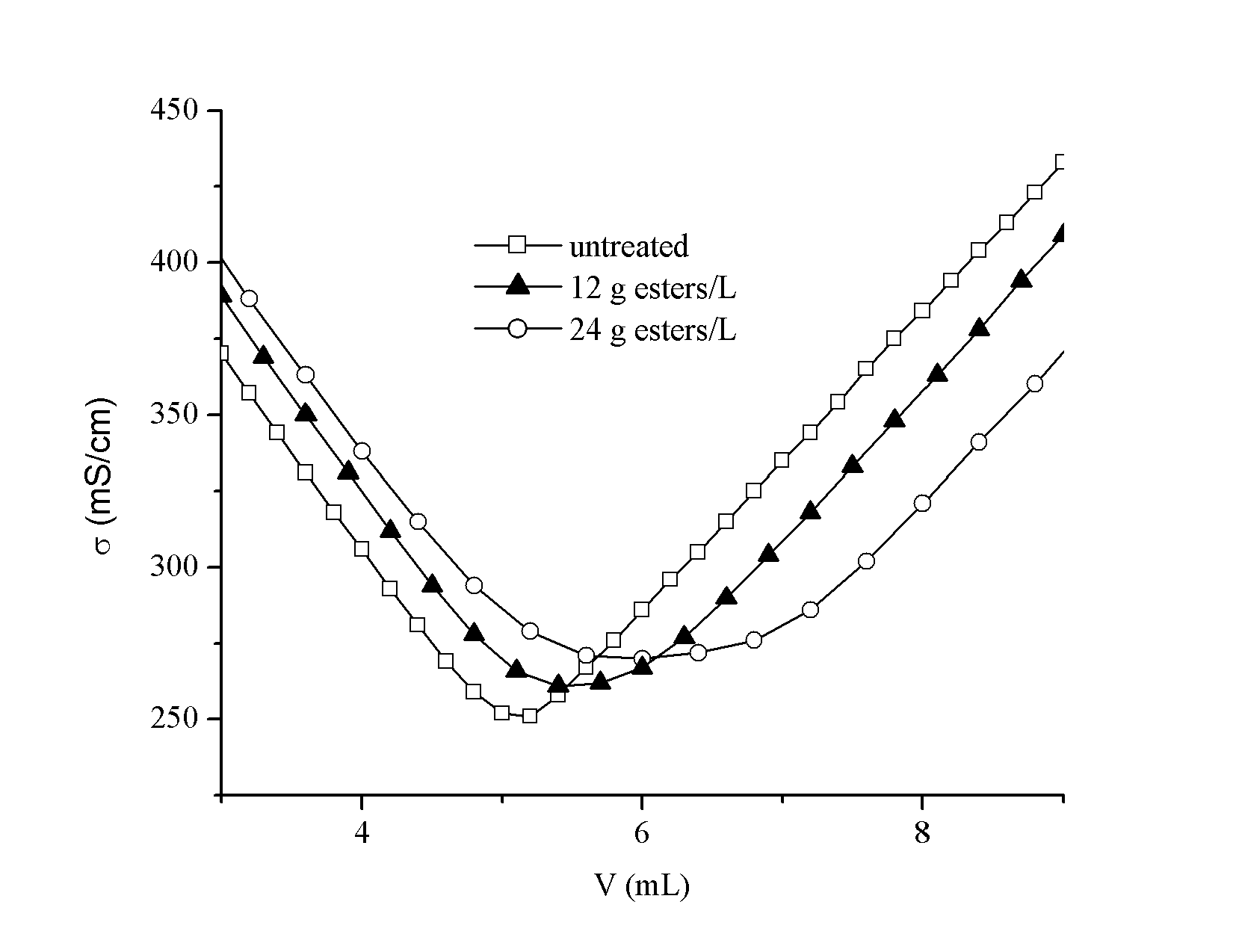 | Figure 2. Conductometric curves of unmodified and modified fibres |
|
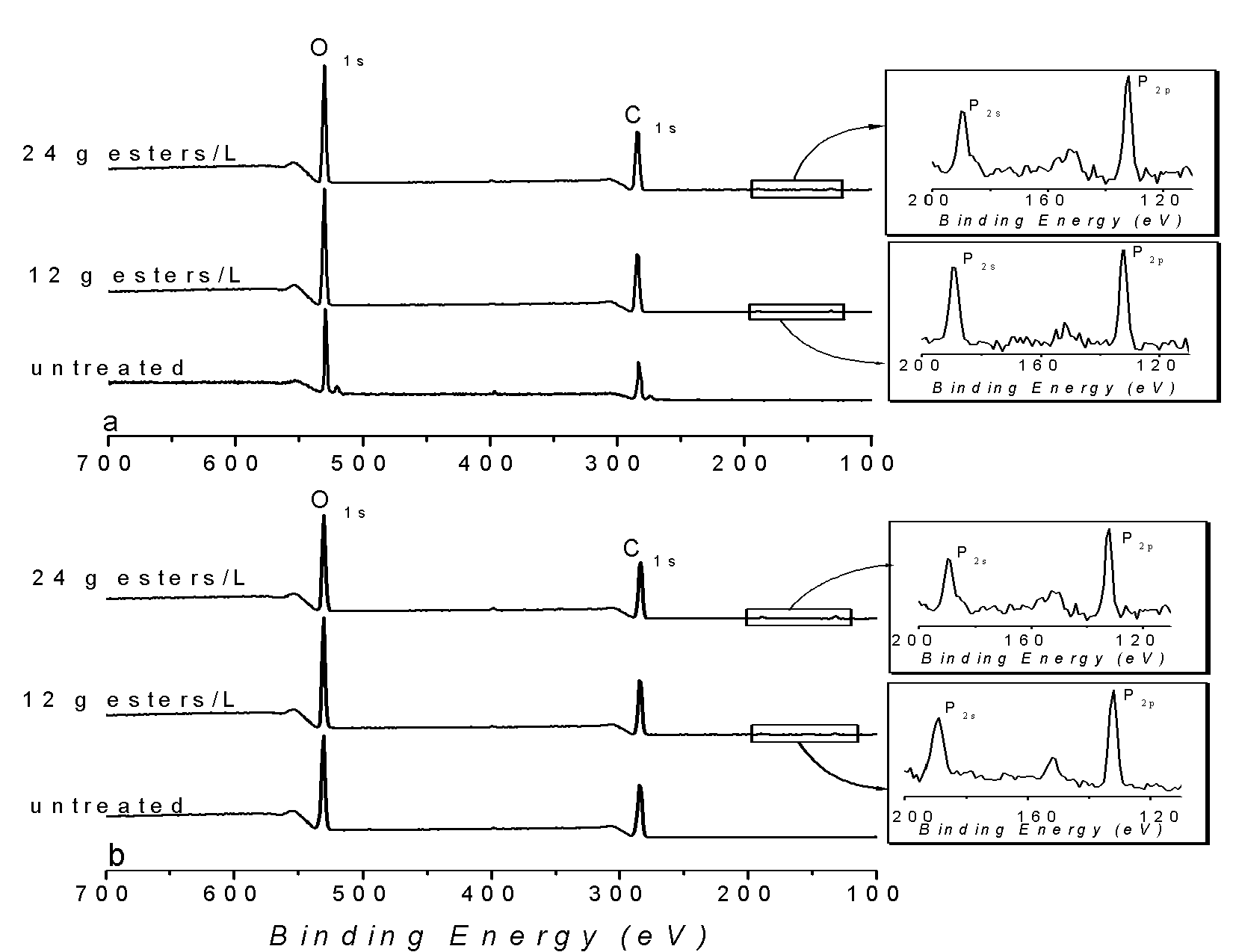 | Figure 3. Full XPS of cellulose (CP and KP) before and after treatment |
|
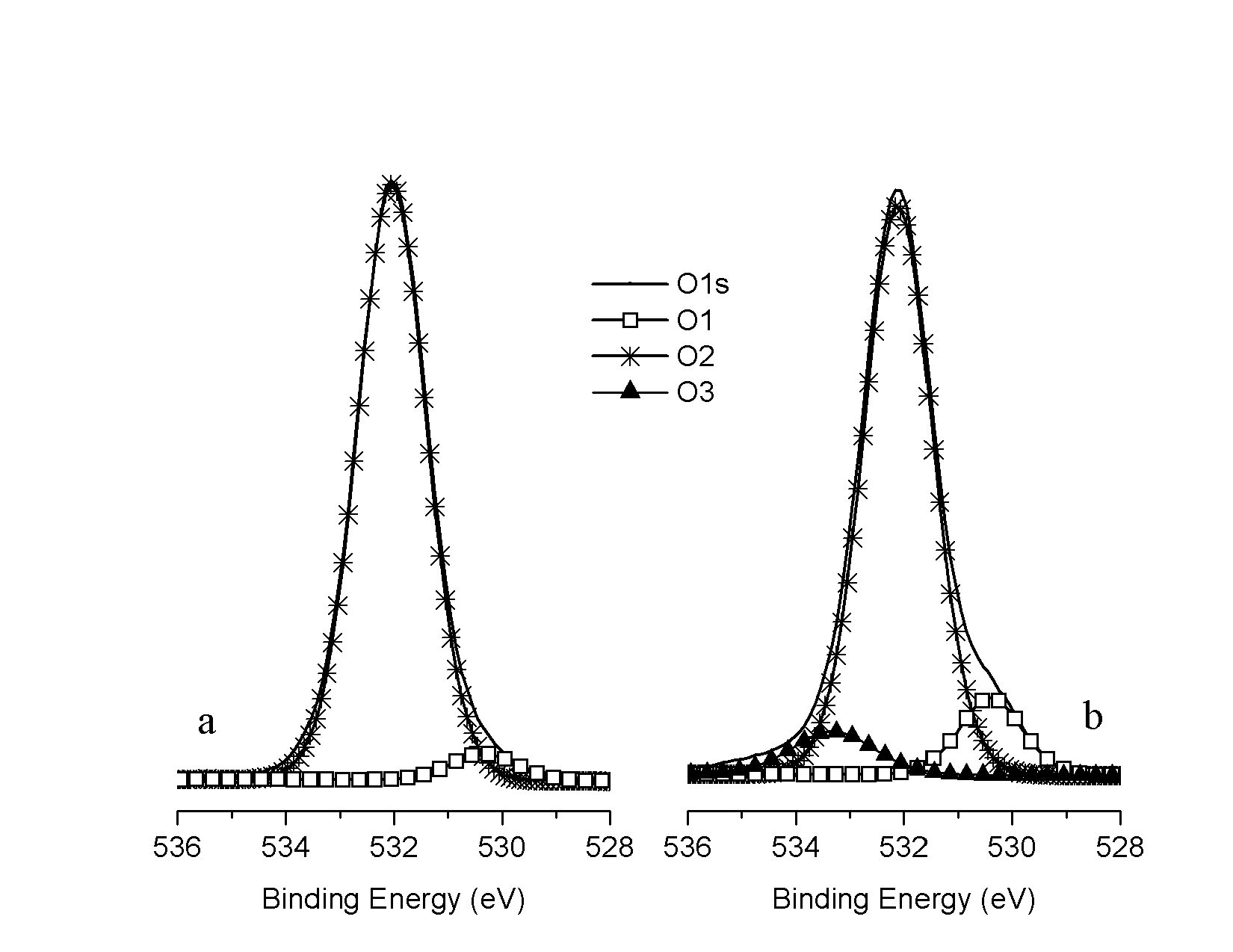 | Figure 4. O1s deconvolution of unmodified (a) and modified (b) fibres |
4. Conclusions
- Results presented above show the presence of phosphate esters on the two studied cellulosic substrates. The preference for monoester overdiester was also noted. This study makes it possible to confirm interactions between cellulose fibres and phosphate esters. Itwas demonstrated that phosphorus could be introduced on fibre surfaces by adding phosphate esters to a fibre suspension. Itwas also revealedthatchemical bonds were formed between fibres and the anti-lint compound. O-P bonds were observed on the fibre surface after multiple washing of the treated fibres. So we can conclude that phosphate esters are linked by chemical bonding and the CellO-P bond is a part of O-P bonds.
ACKNOWLEDGEMENTS
- This research was financially supported the Natural Sciences and Engineering Research Council of Canada (NSERC). The authors would like to thank M. Traoré (undergraduate student) and A. Lejeune and M. Paquin for their technical assistance.
References
| [1] | P. Mangin, A critical review of effect of printing parameters on the linting propensity of paper. Journal of Pulp and Paper Science, 1991. 17(5), 156 -163. |
| [2] | F. Brouillette , D. Morneau, and C.S.H. AG, Additive for reducing paper linting and dusting, European Patent Office, 2006, EP1670988 (A1) |
| [3] | F. Brouillette et al., Evaluation of new lint reduction additives in wood-containing paper manufacturing. Pulp & Paper-Canada, 2006. 107(2), 47-50. |
| [4] | F. Brouillette, Relative efficiency of lintreduction additives in the production of SCB paper - Pilot papermachine trial. ATIP Review, 2010. 62(2), 13-19 |
| [5] | A. Hadj-Bouazza and F. Brouillette, Synthesis of phosphate mono esters and study of theireffect on the reduction of the lintingpropensity of papers. TAPPSA Journal, 2010. 2, 34-37 |
| [6] | J.M.H. Daemen and W. Dankelman, Qualitative and quantitative determinations of mono and dialkylphosphoric acids and their salts by gas chromatography. Journal of Chromatography, 1973. 78, 281-291. |
| [7] | L. Fras et al., Determination of dissociable groups in natural and regenerated cellulose fibers by different titration methods. J. Appl. Polym. Sci., 2004. 92(5), 3186-3195. |
| [8] | L.F. Zemljic et al., Carboxyl groups in pre-treated regenerated cellulose fibres. Cellulose, 2008. 15(5), 681-690. |
| [9] | A. Hirai et al., Phase Separation Behavior in Aqueous Suspensions of Bacterial Cellulose Nanocrystals Prepared by Sulfuric Acid Treatment. Langmuir, 2008. 25(1), 497-502. |
| [10] | W.K. Istone, X-Ray Photoelectron Spectroscopy (XPS), in Surface Analysis of Paper, T.E. Conners and S. Banerjee, Editors. 1995, CRC Press p. 235 - 268. |
| [11] | C. Gaiolas et al., Green chemicals and process to graft cellulose fibers. Journal of Colloid and Interface Science, 2009. 330(2), 298-302. |
| [12] | S. Takeda, et al., Surface OH group governing adsorption properties of metal oxide films. Thin Solid Films, 1999. 339(1-2), 220-224. |
| [13] | C.S.R. Freire, et al., Surface characterization by XPS, contact angle measurements and ToF-SIMS of cellulose fibers partially esterified with fatty acids. Journal of Colloid and Interface Science, 2006. 301(1), 205-209. |
| [14] | B. Ly et al., Surface functionalization of cellulose fibres and their incorporation in renewable polymeric matrices. Composites Science and Technology, 2008. 68(15-16), 3193-3201. |
| [15] | A. Torrisi, XPS study of five fluorinated compounds deposited on calcarenite stone: Part II: Aged samples. Applied Surface Science, 2008. 254(22), 7127-7136. |
| [16] | E.h.B. Ly, et al., Surface functionalization of cellulose by grafting oligoether chains. Materials Chemistry and Physics, 2010. 120(2-3), 438-445. |
| [17] | P.-S. Liu et al., Surface modification of cellulose membranes with zwitterionic polymers for resistance to protein adsorption and platelet adhesion. Journal of Membrane Science, 2010. 350(1-2), 387-394. |
| [18] | P. Rupper et al., Characterization of chars obtained from cellulose treated with phosphoramidate flame retardants. Journal of Analytical and Applied Pyrolysis, 2010. 87(1), 93-98. |
| [19] | M.A. Salim et al., X-ray photoelectron spectroscopy (XPS) and magnetization studies of iron-vanadium phosphate glasses. Journal of Non-Crystalline Solids, 2001. 289(1-3), 185-195. |
| [20] | P.Y. Shih, S.W. Yung, and T.S. Chin, FTIR and XPS studies of P2O5-Na2O-CuO glasses. Journal of Non-Crystalline Solids, 1999. 244(2-3), 211-222. |
| [21] | F. Ahimou et al., XPS analysis of chemical functions at the surface of Bacillus subtilis. Journal of Colloid and Interface Science, 2007. 309(1), 49-55. |
| [22] | A.M. Puziy et al., XPS and NMR studies of phosphoric acid activated carbons. Carbon, 2008. 46(15), 2113-2123. |
| [23] | C.J.P. Boonaert and P.G. Rouxhet, Surface of Lactic Acid Bacteria: Relationships between Chemical Composition and Physicochemical Properties. Appl. Environ. Microbiol., 2000. 66(6), 2548-2554. |
| [24] | P.R. Davies and N.G. Newton, The chemisorption of organophosphorus compounds at an Al(1 1 1) surface. Applied Surface Science, 2001. 181(3-4), 296-306. |
| [25] | A.S. Knyazev et al., Role of phosphates in the promotion of silver catalysts for partial oxidation: I. Structure and properties of phosphates on the surface of polycrystalline silver. Kinetics and Catalysis, 2005. 46(1), 144-150. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML