-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2012; 2(6): 202-209
doi:10.5923/j.materials.20120206.06
Features of the Solid Solution (Mo0.9,Cr0.1)Si2 Formation Depending on the State of Initial Mixture
I. Kud, L. Ieremenko, L. Likhoded, I. Uvarova, D. Zyatkevich
Department for technology of refractory compounds and nanostructured composite coatings, Frantsevich Institute for Problems of Materials Science of NAS of Ukraine, Kyiv, 03142, Ukraine
Correspondence to: D. Zyatkevich, Department for technology of refractory compounds and nanostructured composite coatings, Frantsevich Institute for Problems of Materials Science of NAS of Ukraine, Kyiv, 03142, Ukraine.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The regularities of solid state synthesis of the solid solution (Mo0.9,Cr0.1)Si2 in vacuum have been investigated in the temperature range 400-1200℃ depending on the dispersity and energetic state of the initial powders, namely molybdenum, chromium and silicon. The energetic state of the initial mixture was established to be a determining factor which affects the principal features of solid state interaction whereas an increase in dispersity only influences the temperature of the interaction start. When non-activated initial mixtures and ones mechanically activated in a planetary mill with low number of drum revolutions were used, the solid solution formation proceeded owing to diffusion of silicon into metals through successive formation of lower and higher molybdenum-based silicide phases followed by their interaction. Mechanical activation in a planetary mill with high number of drum revolutions was accompanied by not only decrease in particle size but also changes in the energetic state of the reaction mixture, which resulted in changing the regularities of the solid solution formation. Herein solid solutions on the basis of two higher molybdenum silicide phases, tetra- and hexagonal modifications, were formed with further polymorphic transition of the unstable high temperature hexagonal β-MoSi2 phase into the low temperature tetragonal α-MoSi2 phase. It has been established that temperature of the beginning of interaction decreases by 100℃ as compared with non-activated initial mixtures and temperature of the end of the process depends on the amount of accumulated energy: under low energy mechanical activation the process is complete at 1200℃, while a high energy activation decreases this temperature by 200-400℃ depending on the duration of activation.
Keywords: Silicide, Solid State Reaction, Milling, Nanomaterial
Cite this paper: I. Kud, L. Ieremenko, L. Likhoded, I. Uvarova, D. Zyatkevich, Features of the Solid Solution (Mo0.9,Cr0.1)Si2 Formation Depending on the State of Initial Mixture, American Journal of Materials Science, Vol. 2 No. 6, 2012, pp. 202-209. doi: 10.5923/j.materials.20120206.06.
Article Outline
1. Introduction
- The transition metal silicides refer to refractory compounds with melting temperature close to that of borides and carbides. Their high thermal and chemical stabilities remain up to high temperatures, which makes them promising as materials for refractory coatings, various parts for aircraft and space engineering as well as for metallurgical and chemical industry[1-5]. Among all of the silicides, MoSi2 based materials are most known for high temperature application. They typically have good mechanical properties and high heat and oxidation resistance at high (above 900℃) temperatures. However, a number of shortcomings at lower temperatures block their commercial application. A key shortcoming is drastic oxidation and disintegration at 800-900℃. Some positive results concerning the avoidance of the effect of the low temperature oxidation have been achieved lately. In particular, composite materials with ceramic additives from ZrO2, Al2O3, SiC, etc. have been created. Also, a promisingsolution of this problem consists in using solid solutions of molybdenum silicides added with chromium, niobium, titanium, tungsten and other refractory elements. The use of chromium as a basic additive to MoSi2 increases the oxidation resistance of this material in the temperature range 600-900℃[6-9].Investigations into the structure and physicochemical properties in complex silicide systems such as Cr-Me-Si, where Ме stands for Ti, Ta, Re, and Mn[10-12], have shown that solid solutions on the basis of silicides exhibit better physicochemical properties compared to the related individual silicides. The broad region of the existence of solid solutions, within which the crystalline and electron structures are the same, guarantees a high reproducibility of the operation parameters. Herein solid solutions on the basis of higher silicide phases have the most stable properties. The use of nanosized powders of solid solutions of silicides as materials for coatings and volumetric heaters markedly increases their high temperature resistance due to the formation of fine-grained protective film of scale. In spite of good enough clarification of mechanical alloying in various systems[13-15] as well as the regularities of formation of nanosized individual silicides, in particular silicides of molybdenum and titanium, via high energy mechanical treatment[16-22], the available information on production of nanopowders of solid solutions of refractory compounds, especially silicides, is poor. This work is devoted to investigation into the regularities of the solid solution (Mo0.9,Cr0.1)Si2 formation through a solid state synthesis depending on the dispersity and energetic state of the initial mixture after milling.
2. Experimental
- In this study, the following microsized powders were used as initial components: silicon (99.998 % Si, Ssp=2.2 m2/g produced by the Svitlovodsk Plant of Hard Alloys, Ukrainе); molybdenum PM 99.95 (99.95 % Mo, Ssp=0.5 m2/g, Firm “Polema”, Russia), and electrolytic chromium PERCr-1/280 (98.7% Cr, Ssp = 0.2 m2/g, “Polema”, Russia).According to the literature [10], the region of the solid solution (Mo0.9,Cr0.1)Si2 existence spreads up to 46 mol% CrSi2. In this work, therefore, the initial mixture was calculated in view of production of the solid solution (Mo0.9,Cr0.1)Si2, namely (mass%): 58.45 Mo; 3.52 Cr, and 38.03 Si. Besides, some extra silicon was added, whose amount was selected tentatively to compensate losses of silicon during the mill discharge as the lightest and most inclined to oxidation element among the other components. A homogeneous component distribution was reached via milling in the steel drum of planetary mill «Pulverizette-6» in an ethanol medium. The powder particle size did not exceed 10 µm.To investigate the effect of the dispersity and the energetic state of the initial mixtures on the solid solution (Mo0.9,Cr0.1)Si2 formation, different conditions of mechanical activation were selected which were provided by different types of planetary mills used. In particular, mechanical activation was performed in a high-energy planetary mill «AIR-0.015» («Gidrotsvetmet», Russia) in an argon medium at a planetary disk revolution number of 548 rpm with an acceleration of 25 g as well as in a planetary mill «Pulverizette-6» at a disk revolution number of 200 rpm. The mass powder/balls ratio was 1:10. Milling in «Pulverizette-6» was performed under the conditions, which provided a decrease in the particle size of the initial powder with no change in its composition. Milling in «AIR-0.015» was conducted using an impulse regime, which made it possible to avoid an uncontrolled SHS (self-propagating high temperature synthesis) reaction. The amount of the initial mixture was the same in all experiments and equal to 50 g. The steel drums and 10 mm milling balls were prior charged with silicon to prevent contamination of the products with the reactor material. The energy transferred to the powder under milling was estimated on the basis of the specific energy, that is, the energy acquired by 1 g powder after one ball collision [23-24], taking into account the time of milling. A criterion for choice of the milling time was minimal change in the phase composition of initial mixture.Solid state synthesis of both non-activated and previously mechanically activated reaction mixtures (3 g samples pressed under 0.5 MPа) was conducted in a vacuum furnace SNV-1.3,1/20-I1 at ~ 1·10–3 Pа in the temperature range 400-1200℃ and 2 h isothermal holding. The average rate of temperature rise to the isothermal holding was 10 K/min. The synthesis products were examined by an XRD analysis using a diffractometer DRON-3 under filtered Co radiation. The angles were determined with an error of ± 0.05 deg. The size of crystallites was calculated using the Sherrer formula. The phases were identified following the powder diffraction database file ICDD-JCPDS PDF-2. The error of the lattice parameter calculation was ± 0.001 nm. The specific surface area was determined using thermal desorption of nitrogen with an error of 5-10 %. The particle size and elemental composition of the powders produced by a low temperature synthesis were determined with help of a transmission electron microscope JEM 2100 S (Japan). Standard techniques of chemical analysis were used for determination of the elemental composition of the final product and estimation of the amount of iron milled into it.
3. Results and Discussion
3.1. High Temperature Interaction of Non-activated Powders of Molybdenum, Chromium and Silicon in Vacuum
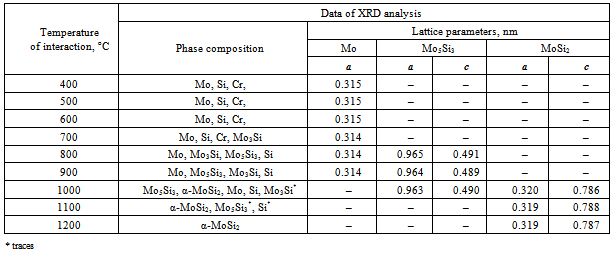
- The regularities of the (Mo0.9,Cr0.1)Si2 formation in the course of solid state interaction of the initial components in the temperature range 400-1200℃ were investigated at an isothermal holding of 2 h. The corresponding XRD findings for the synthesis products are listed in Table 1.These data evidence that the solid state interaction of the initial components begins at 700℃, when the lower molybdenum silicide appears, whose reflection intensities change extremely with a maximum at 800℃. The interaction is accompanied with reduction in the molybdenum lattice parameter, which may be assigned to diffusion processes and dissolution of chromium in molybdenum (atomic radius of chromium is smaller than that of molybdenum by 10 %). Below 700℃ no phase transition is fixed and the reflection intensity is unchanged.The further temperature rise is accompanied with the beginning of formation at 800℃ of the tetragonal phase Мо5Si3, which is present in the interaction products up to 1100℃. At 1000℃ the higher molybdenum silicide of the tetragonal modification, α-МоSi2, is formed. Thus, in the temperature range 1000-1100℃ the basic products are Мо5Si3 and α-МоSi2 along with traces of Si, whereas at 1200℃ only α-МоSi2 is identified. The change in the α-МоSi2 lattice parameters (а decreases and с markedly increases) indicates the formation of the interstitial solid solution (Mox,Cry)Si2. The data of chemical analysis demonstrate high reproducibility of the elemental composition (58.5 Мо; 3.74 Cr; 37.6 Si, mass%), which practically corresponds to the calculated formula (Mo0.9,Cr0.1)Si2; contamination with iron does not exceed 0.1 mass%. The absence of chromium silicide phases in the temperature range studied draws a conclusion that the solid state interaction proceeds through successive formation of interstitial solid solutions on the basis of molybdenum silicide phases owing to the dissolution of chromium. This is well consistent with the equilibrium diagrams of the Mo-Cr-Si system[10], according to which the isostructural silicides Cr3Si-Mo3Si and Cr5Si3-Mo5Si3 form a continuous series of solid solutions.
3.2. Low Temperature Interaction of Previously Mechanically Activated Powders of Molybdenum, Chromium and Silicon
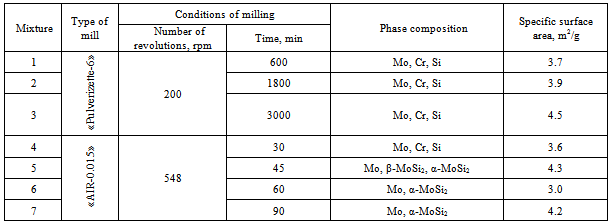
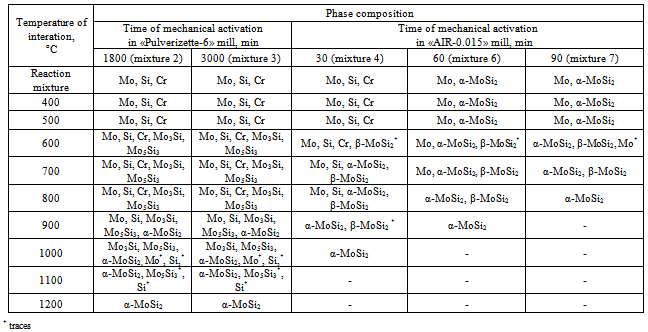
- The effect of the dispersity of the initial mixture and the energy acquired by it on the regularities of the solid solution (Mox,Cry)Si2 formation was studied using an XRD and TEM analyses. Table 2 lists the XRD data and the results of determination of the specific surface energy upon milling.As seen, a high-energy milling leads to the powder refining, which is accompanied by change in the diffraction peaks and the phase composition of reaction mixture. For mixtures 1-3 subjected to mechanical activation in the mill «Pulverizette-6», prolongation of milling from 600 to 3000 min results in changing the peak intensity and broadening the peaks for all of the components used. First of all, this may be connected with powder refining: the specific surface area monotonously increases from 3.7 m2/g (600 min milling) tо 4.5 m2/g (3000 min milling). The dynamics of changing in the XRD patterns from the reaction mixtures subjected to milling in the planetary mill «AIR-0.015» demonstrates the significant influence of the acquired energy on the reactivity (Fig. 1). The 30 min milling does not cause any change in the phase composition of reaction mixture; only some distortion of the diffraction peaks is observed, which is particularly characteristic for silicon, which is more brittle compared to molybdenum and chromium and is therefore affected by mechanical processing to a greater extent. Its energetic state is more strained owing to refining and disordering of the crystalline lattice.Prolongation of milling to 45 min leads to change in the phase composition. The molybdenum peaks markedly broaden, and reflections from silicon and chromium are absent. In addition, nuclei of molybdenum disilicide of two modifications, namely low temperature α-MoSi2 and high temperature β-MoSi2, appear practically in equal quantity.
|
|
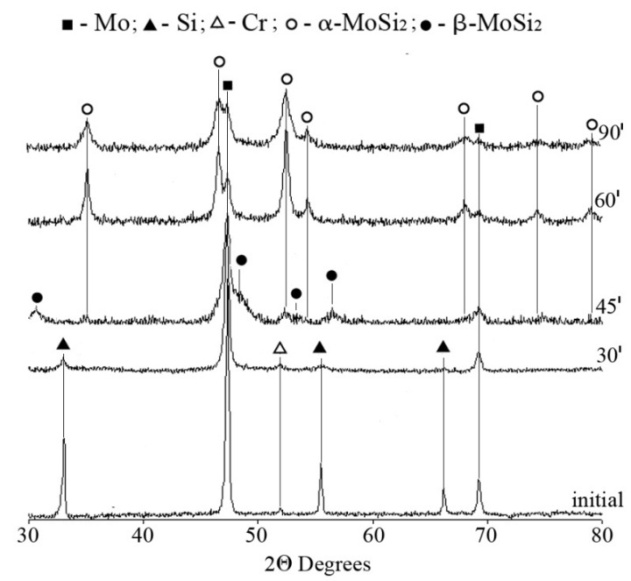 | Figure 1. XRD patterns from the reaction mixture before (initial) and after milling for 30, 45, 60, and 90 min in the planetary mill «AIR-0.015» |
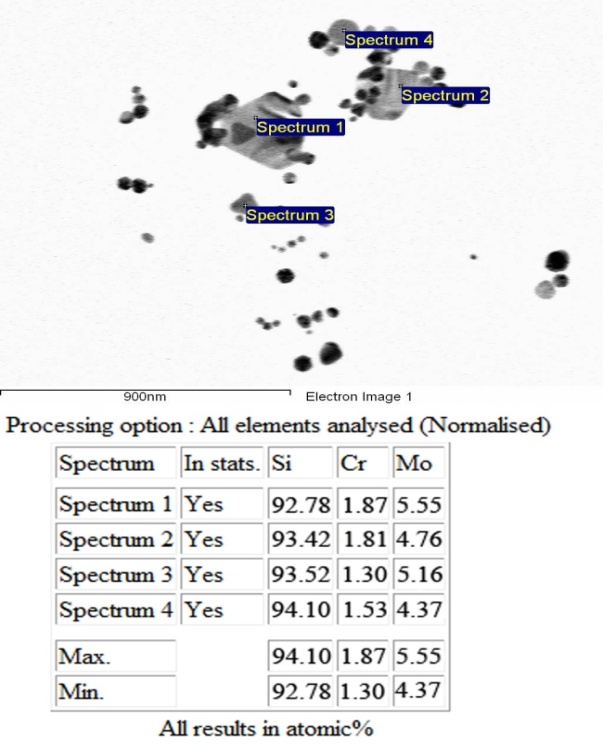 | Figure 2. Elemental composition of the light phase separated from air during discharge of the planetary mill «AIR-0.015» |
 | Figure 3. The surface of agglomerate (а) and maps of distribution of silicon (b), chromium (c) and molybdenum (d) over it for the reaction mixture mechanically activated for 90 min |
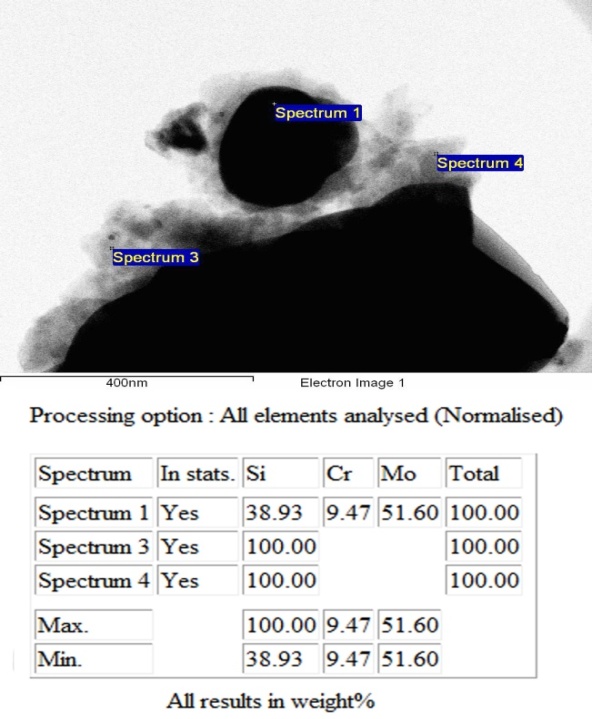 | Figure 4. Elemental composition of the activated reaction mixture |
|
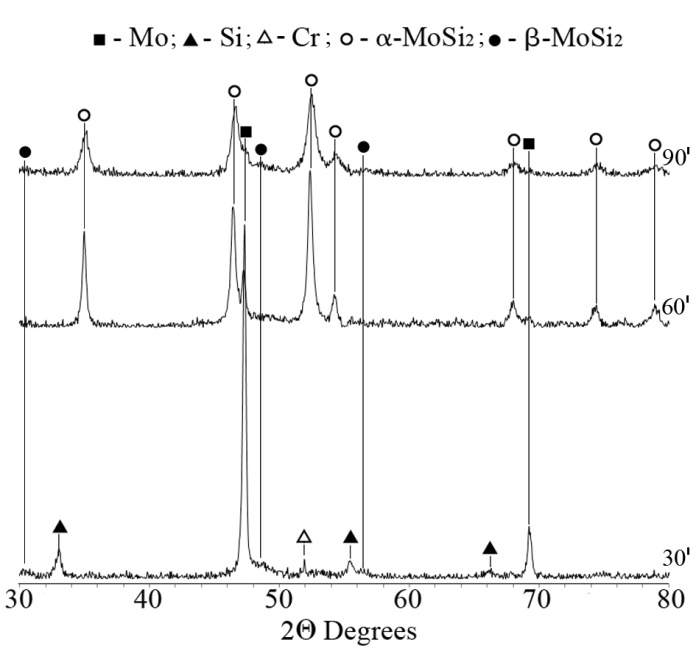 | Figure 5. XRD patterns from the products of solid state interaction at 600℃ of mixtures mechanically activated for 30, 60 and 90 min |
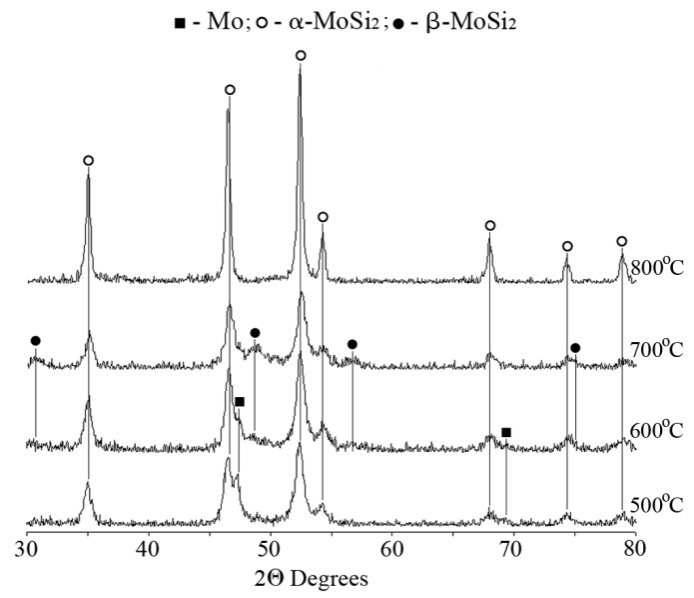 | Figure 6. XRD patterns from the products of solid state interaction at different temperatures of the reaction mixture mechanically activated for 90 min |
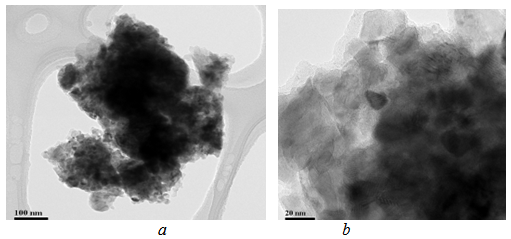 | Figure 7. TEM micrographs of the solid solution (Mo0.9,Cr0.1)Si2 particles produced at 800℃ |
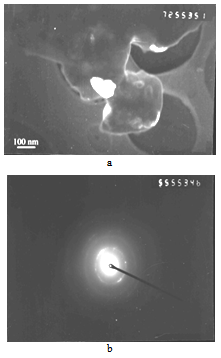 | Figure 8. TEM micrographs of the solid solution (Mo0.9,Cr0.1)Si2 powder produced at 800℃ (а) and electron-diffraction pattern of a particle (b) |
4. Conclusions
- The energy acquired by the initial reaction mixture during mechanical activation was established to be a determining factor among the others affecting the process of solid state interaction, whereas the role of the dispersity exhibits itself only in the change in the interaction start temperature. Formation of the solid solution (Mo0.9,Cr0.1)Si2 through solid state interaction of non-activated initial powders occurs owing to diffusion processes via intermediate stages of formation of solid solutions based on lower and higher molybdenum silicide phases and their subsequent interaction.Mechanical activation in a planetary mill «Pulverizette-6» is accompanied with refining the initial components; the energy acquired by them permits a decrease in the temperature of the interaction start by 100℃; herein the regularities of formation of dissipative intermediate phases and the final temperature of the solid solution formation, 1200℃, are the same as for non-activated powders. Mechanical activation in a high-energy mill «АІR-0.015» allows one to produce high reactivity mixtures, which intensifies the solid state interaction. Under the following heat treatment, solid solutions on the basis of higher molybdenum silicide phases of two modifications, tetragonal α-MoSi2 and hexagonal β-MoSi2, are formed with further transformation of the unstable high temperature β-MoSi2 phase into the low temperature α-MoSi2 phase.
ACKNOWLEDGEMENTS
- The work was fulfilled with the support of the STCU under Project 4182.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML